 Found 56 hits with Last Name = 'ge' and Initial = 'zm'
Found 56 hits with Last Name = 'ge' and Initial = 'zm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50115808

(CHEMBL61616 | Dimethyl-[2-(pyridin-3-yloxy)-ethyl]...)Show InChI InChI=1S/C9H14N2O/c1-11(2)6-7-12-9-4-3-5-10-8-9/h3-5,8H,6-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Affinity to alpha-4-beta-2 AChR |
Bioorg Med Chem Lett 16: 2013-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.073
BindingDB Entry DOI: 10.7270/Q2WD41TH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50115825
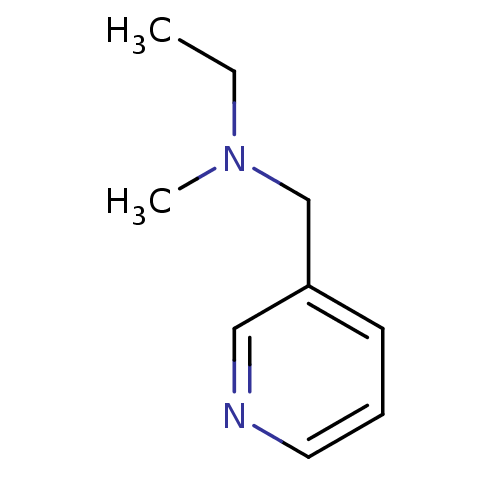
(CHEMBL60792 | Ethyl-methyl-pyridin-3-ylmethyl-amin...)Show InChI InChI=1S/C9H14N2/c1-3-11(2)8-9-5-4-6-10-7-9/h4-7H,3,8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Affinity to alpha-4-beta-2 AChR |
Bioorg Med Chem Lett 16: 2013-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.073
BindingDB Entry DOI: 10.7270/Q2WD41TH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50120586
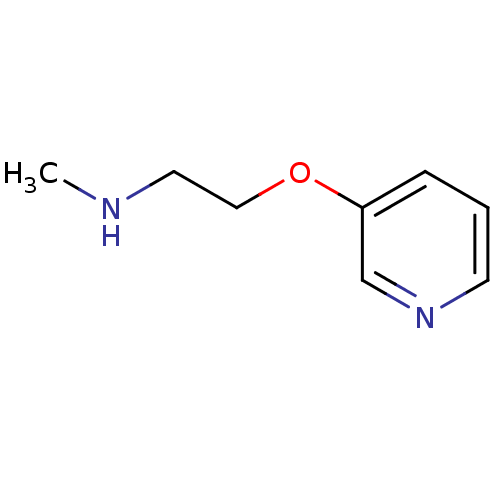
(CHEMBL112221 | Methyl-[2-(pyridin-3-yloxy)-ethyl]-...)Show InChI InChI=1S/C8H12N2O/c1-9-5-6-11-8-3-2-4-10-7-8/h2-4,7,9H,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Affinity to alpha-4-beta-2 AChR |
Bioorg Med Chem Lett 16: 2013-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.073
BindingDB Entry DOI: 10.7270/Q2WD41TH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50336020
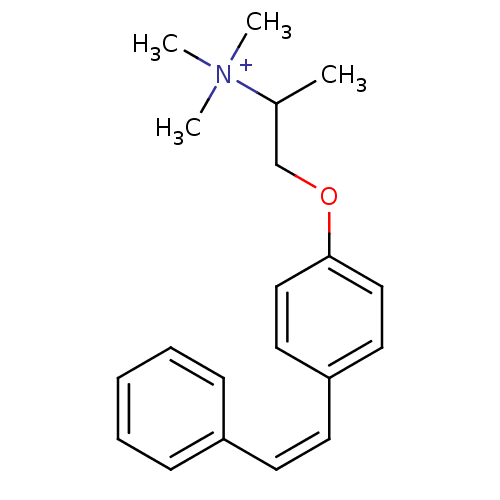
(CHEMBL1669092 | N,N,N-trimethyl-1-(4-styrylphenoxy...)Show InChI InChI=1S/C20H26NO/c1-17(21(2,3)4)16-22-20-14-12-19(13-15-20)11-10-18-8-6-5-7-9-18/h5-15,17H,16H2,1-4H3/q+1/b11-10- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Binding affinity to alpha7 nAChR |
Bioorg Med Chem Lett 21: 940-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.052
BindingDB Entry DOI: 10.7270/Q2M045Q6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50138489
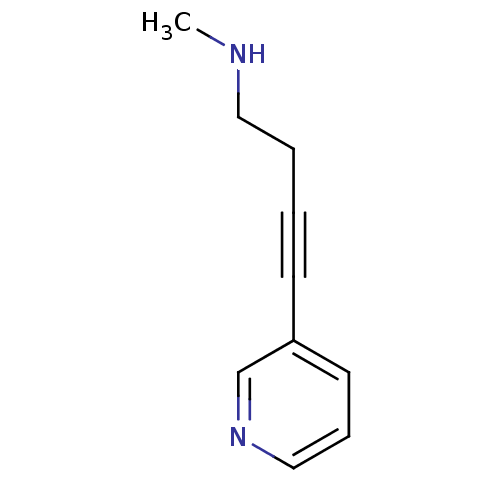
(CHEMBL54860 | Methyl-(4-pyridin-3-yl-but-3-ynyl)-a...)Show InChI InChI=1S/C10H12N2/c1-11-7-3-2-5-10-6-4-8-12-9-10/h4,6,8-9,11H,3,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Affinity to alpha-4-beta-2 AChR |
Bioorg Med Chem Lett 16: 2013-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.073
BindingDB Entry DOI: 10.7270/Q2WD41TH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50138494
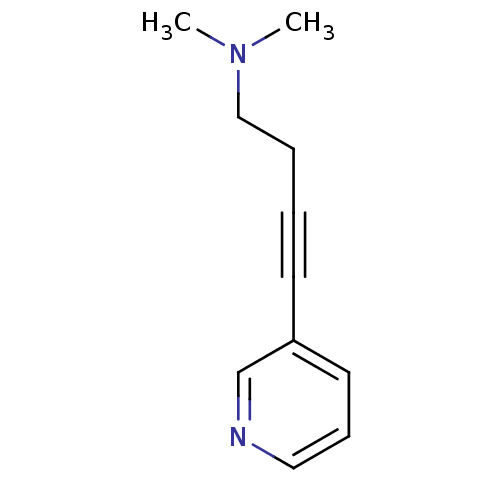
(CHEMBL150893 | Dimethyl-(4-pyridin-3-yl-but-3-ynyl...)Show InChI InChI=1S/C11H14N2/c1-13(2)9-4-3-6-11-7-5-8-12-10-11/h5,7-8,10H,4,9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Affinity to alpha-4-beta-2 AChR |
Bioorg Med Chem Lett 16: 2013-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.073
BindingDB Entry DOI: 10.7270/Q2WD41TH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50119559
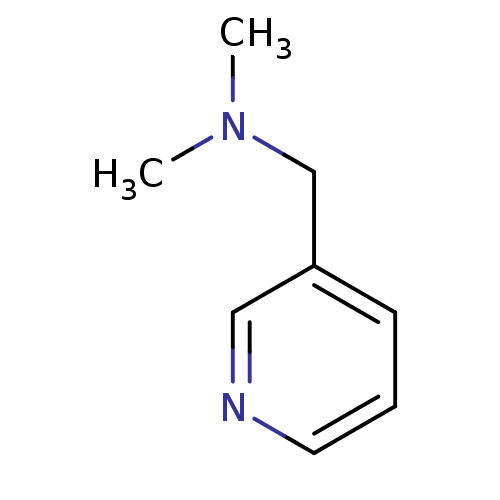
(CHEMBL331904 | Dimethyl-pyridin-3-ylmethyl-amine |...)Show InChI InChI=1S/C8H12N2/c1-10(2)7-8-4-3-5-9-6-8/h3-6H,7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Affinity to alpha-4-beta-2 AChR |
Bioorg Med Chem Lett 16: 2013-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.073
BindingDB Entry DOI: 10.7270/Q2WD41TH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50138487
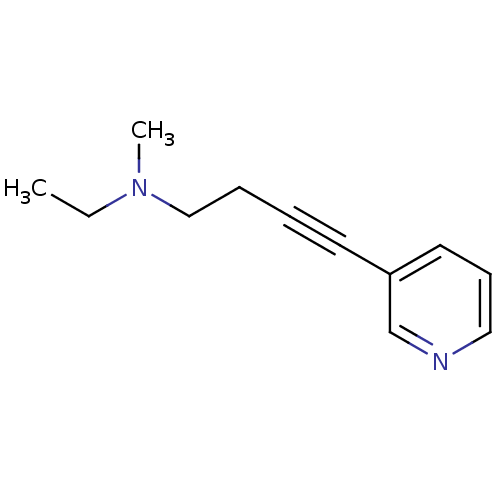
(CHEMBL150948 | Ethyl-methyl-(4-pyridin-3-yl-but-3-...)Show InChI InChI=1S/C12H16N2/c1-3-14(2)10-5-4-7-12-8-6-9-13-11-12/h6,8-9,11H,3,5,10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Affinity to alpha-4-beta-2 AChR |
Bioorg Med Chem Lett 16: 2013-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.073
BindingDB Entry DOI: 10.7270/Q2WD41TH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50138486
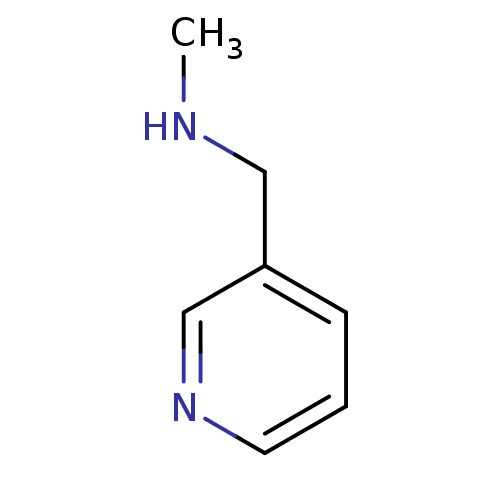
(CHEMBL357101 | Methyl-pyridin-3-ylmethyl-amine | N...)Show InChI InChI=1S/C7H10N2/c1-8-5-7-3-2-4-9-6-7/h2-4,6,8H,5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Affinity to alpha-4-beta-2 AChR |
Bioorg Med Chem Lett 16: 2013-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.073
BindingDB Entry DOI: 10.7270/Q2WD41TH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit beta-2
(Homo sapiens (Human)) | BDBM50336020

(CHEMBL1669092 | N,N,N-trimethyl-1-(4-styrylphenoxy...)Show InChI InChI=1S/C20H26NO/c1-17(21(2,3)4)16-22-20-14-12-19(13-15-20)11-10-18-8-6-5-7-9-18/h5-15,17H,16H2,1-4H3/q+1/b11-10- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Binding affinity to beta2 nAChR |
Bioorg Med Chem Lett 21: 940-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.052
BindingDB Entry DOI: 10.7270/Q2M045Q6 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259656
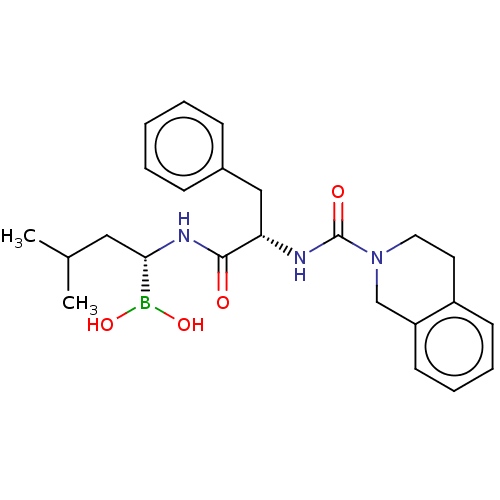
(CHEMBL4089402)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCc2ccccc2C1)B(O)O |r| Show InChI InChI=1S/C24H32BN3O4/c1-17(2)14-22(25(31)32)27-23(29)21(15-18-8-4-3-5-9-18)26-24(30)28-13-12-19-10-6-7-11-20(19)16-28/h3-11,17,21-22,31-32H,12-16H2,1-2H3,(H,26,30)(H,27,29)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.000200 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259645
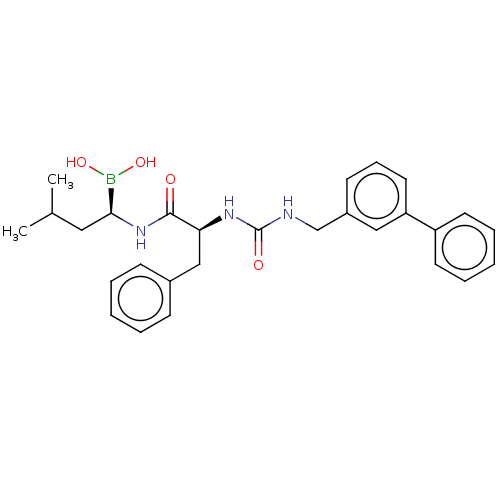
(CHEMBL4070336)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(c1)-c1ccccc1)B(O)O |r| Show InChI InChI=1S/C28H34BN3O4/c1-20(2)16-26(29(35)36)32-27(33)25(18-21-10-5-3-6-11-21)31-28(34)30-19-22-12-9-15-24(17-22)23-13-7-4-8-14-23/h3-15,17,20,25-26,35-36H,16,18-19H2,1-2H3,(H,32,33)(H2,30,31,34)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259657

(CHEMBL4068221)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N(C)Cc1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-17(2)14-21(24(30)31)26-22(28)20(15-18-10-6-4-7-11-18)25-23(29)27(3)16-19-12-8-5-9-13-19/h4-13,17,20-21,30-31H,14-16H2,1-3H3,(H,25,29)(H,26,28)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259647
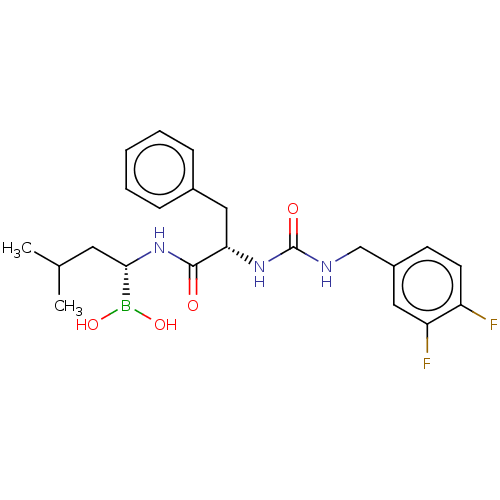
(CHEMBL4100727)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccc(F)c(F)c1)B(O)O |r| Show InChI InChI=1S/C22H28BF2N3O4/c1-14(2)10-20(23(31)32)28-21(29)19(12-15-6-4-3-5-7-15)27-22(30)26-13-16-8-9-17(24)18(25)11-16/h3-9,11,14,19-20,31-32H,10,12-13H2,1-2H3,(H,28,29)(H2,26,27,30)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259659

(CHEMBL4076838)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(c1)[N+]([O-])=O)B(O)O |r| Show InChI InChI=1S/C22H29BN4O6/c1-15(2)11-20(23(30)31)26-21(28)19(13-16-7-4-3-5-8-16)25-22(29)24-14-17-9-6-10-18(12-17)27(32)33/h3-10,12,15,19-20,30-31H,11,13-14H2,1-2H3,(H,26,28)(H2,24,25,29)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259642

(CHEMBL4077037)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(C)c1C)B(O)O |r| Show InChI InChI=1S/C24H34BN3O4/c1-16(2)13-22(25(31)32)28-23(29)21(14-19-10-6-5-7-11-19)27-24(30)26-15-20-12-8-9-17(3)18(20)4/h5-12,16,21-22,31-32H,13-15H2,1-4H3,(H,28,29)(H2,26,27,30)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259655
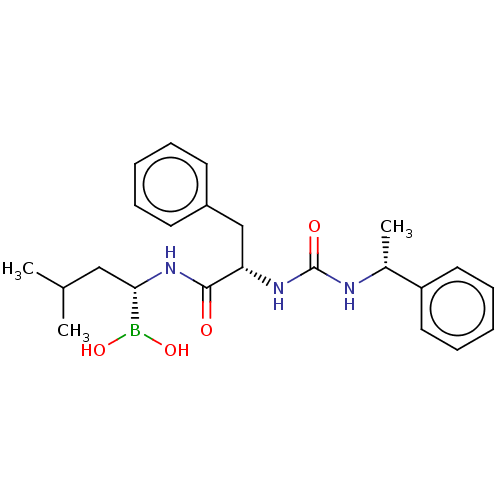
(CHEMBL4080306)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N[C@H](C)c1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-16(2)14-21(24(30)31)27-22(28)20(15-18-10-6-4-7-11-18)26-23(29)25-17(3)19-12-8-5-9-13-19/h4-13,16-17,20-21,30-31H,14-15H2,1-3H3,(H,27,28)(H2,25,26,29)/t17-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259641
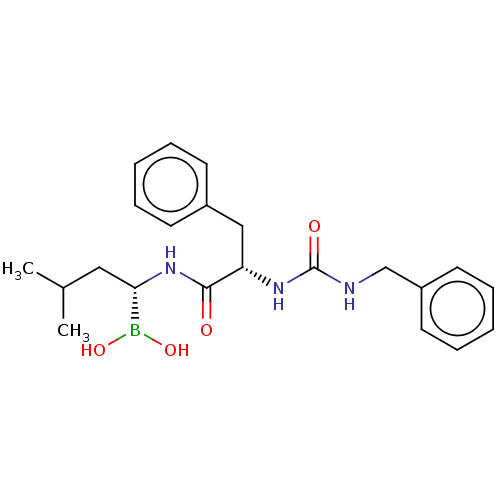
(CHEMBL484701)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccccc1)B(O)O |r| Show InChI InChI=1S/C22H30BN3O4/c1-16(2)13-20(23(29)30)26-21(27)19(14-17-9-5-3-6-10-17)25-22(28)24-15-18-11-7-4-8-12-18/h3-12,16,19-20,29-30H,13-15H2,1-2H3,(H,26,27)(H2,24,25,28)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259646
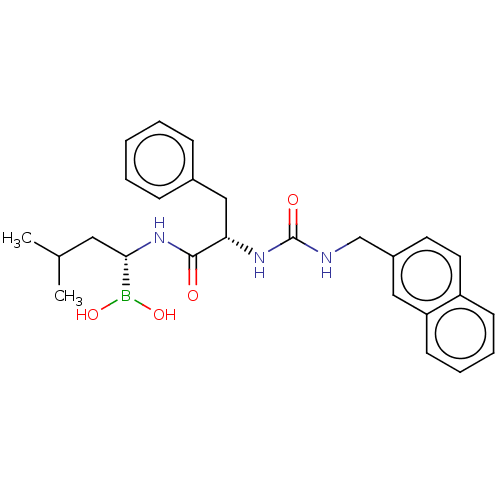
(CHEMBL4075225)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccc2ccccc2c1)B(O)O |r| Show InChI InChI=1S/C26H32BN3O4/c1-18(2)14-24(27(33)34)30-25(31)23(16-19-8-4-3-5-9-19)29-26(32)28-17-20-12-13-21-10-6-7-11-22(21)15-20/h3-13,15,18,23-24,33-34H,14,16-17H2,1-2H3,(H,30,31)(H2,28,29,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259649
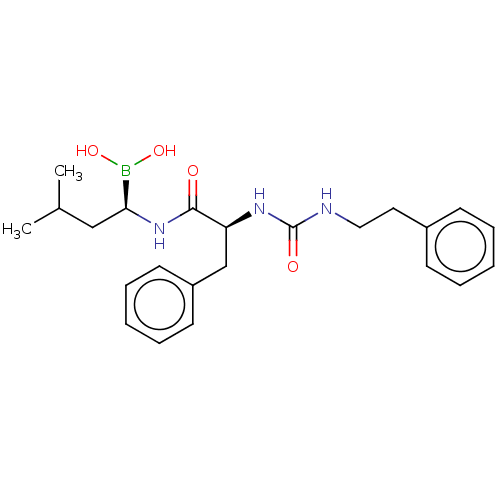
(CHEMBL4077282)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCCc1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-17(2)15-21(24(30)31)27-22(28)20(16-19-11-7-4-8-12-19)26-23(29)25-14-13-18-9-5-3-6-10-18/h3-12,17,20-21,30-31H,13-16H2,1-2H3,(H,27,28)(H2,25,26,29)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259658

(CHEMBL4096371)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccco1)B(O)O |r| Show InChI InChI=1S/C20H28BN3O5/c1-14(2)11-18(21(27)28)24-19(25)17(12-15-7-4-3-5-8-15)23-20(26)22-13-16-9-6-10-29-16/h3-10,14,17-18,27-28H,11-13H2,1-2H3,(H,24,25)(H2,22,23,26)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259656

(CHEMBL4089402)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCc2ccccc2C1)B(O)O |r| Show InChI InChI=1S/C24H32BN3O4/c1-17(2)14-22(25(31)32)27-23(29)21(15-18-8-4-3-5-9-18)26-24(30)28-13-12-19-10-6-7-11-20(19)16-28/h3-11,17,21-22,31-32H,12-16H2,1-2H3,(H,26,30)(H,27,29)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of 20S proteasome in human HL60 cells using Z-nLPnLDaminoluciferin as substrate after 2 hrs by fluorescence analy... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259657

(CHEMBL4068221)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N(C)Cc1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-17(2)14-21(24(30)31)26-22(28)20(15-18-10-6-4-7-11-18)25-23(29)27(3)16-19-12-8-5-9-13-19/h4-13,17,20-21,30-31H,14-16H2,1-3H3,(H,25,29)(H,26,28)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of 20S proteasome in human HL60 cells using Z-nLPnLDaminoluciferin as substrate after 2 hrs by fluorescence analy... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of 20S proteasome in human HL60 cells using Z-nLPnLDaminoluciferin as substrate after 2 hrs by fluorescence analy... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259644
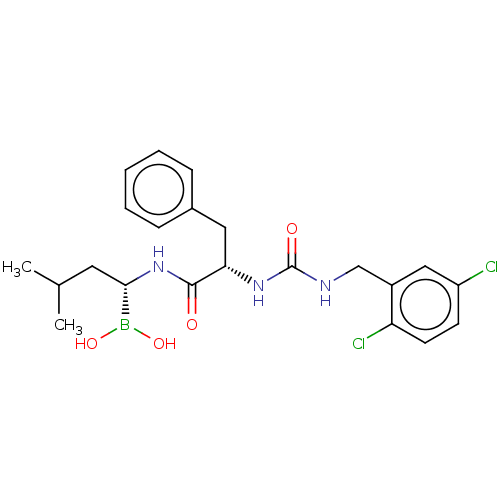
(CHEMBL4091983)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cc(Cl)ccc1Cl)B(O)O |r| Show InChI InChI=1S/C22H28BCl2N3O4/c1-14(2)10-20(23(31)32)28-21(29)19(11-15-6-4-3-5-7-15)27-22(30)26-13-16-12-17(24)8-9-18(16)25/h3-9,12,14,19-20,31-32H,10-11,13H2,1-2H3,(H,28,29)(H2,26,27,30)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259643
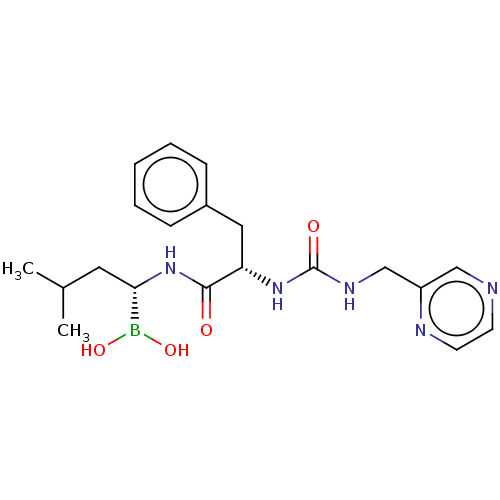
(CHEMBL4065491)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cnccn1)B(O)O |r| Show InChI InChI=1S/C20H28BN5O4/c1-14(2)10-18(21(29)30)26-19(27)17(11-15-6-4-3-5-7-15)25-20(28)24-13-16-12-22-8-9-23-16/h3-9,12,14,17-18,29-30H,10-11,13H2,1-2H3,(H,26,27)(H2,24,25,28)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259655

(CHEMBL4080306)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N[C@H](C)c1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-16(2)14-21(24(30)31)27-22(28)20(15-18-10-6-4-7-11-18)26-23(29)25-17(3)19-12-8-5-9-13-19/h4-13,16-17,20-21,30-31H,14-15H2,1-3H3,(H,27,28)(H2,25,26,29)/t17-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of 20S proteasome in human HL60 cells using Z-nLPnLDaminoluciferin as substrate after 2 hrs by fluorescence analy... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259658

(CHEMBL4096371)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccco1)B(O)O |r| Show InChI InChI=1S/C20H28BN3O5/c1-14(2)11-18(21(27)28)24-19(25)17(12-15-7-4-3-5-8-15)23-20(26)22-13-16-9-6-10-29-16/h3-10,14,17-18,27-28H,11-13H2,1-2H3,(H,24,25)(H2,22,23,26)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259647

(CHEMBL4100727)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccc(F)c(F)c1)B(O)O |r| Show InChI InChI=1S/C22H28BF2N3O4/c1-14(2)10-20(23(31)32)28-21(29)19(12-15-6-4-3-5-7-15)27-22(30)26-13-16-8-9-17(24)18(25)11-16/h3-9,11,14,19-20,31-32H,10,12-13H2,1-2H3,(H,28,29)(H2,26,27,30)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259646

(CHEMBL4075225)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccc2ccccc2c1)B(O)O |r| Show InChI InChI=1S/C26H32BN3O4/c1-18(2)14-24(27(33)34)30-25(31)23(16-19-8-4-3-5-9-19)29-26(32)28-17-20-12-13-21-10-6-7-11-22(21)15-20/h3-13,15,18,23-24,33-34H,14,16-17H2,1-2H3,(H,30,31)(H2,28,29,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259642

(CHEMBL4077037)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(C)c1C)B(O)O |r| Show InChI InChI=1S/C24H34BN3O4/c1-16(2)13-22(25(31)32)28-23(29)21(14-19-10-6-5-7-11-19)27-24(30)26-15-20-12-8-9-17(3)18(20)4/h5-12,16,21-22,31-32H,13-15H2,1-4H3,(H,28,29)(H2,26,27,30)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of 20S proteasome in human HL60 cells using Z-nLPnLDaminoluciferin as substrate after 2 hrs by fluorescence analy... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259659

(CHEMBL4076838)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(c1)[N+]([O-])=O)B(O)O |r| Show InChI InChI=1S/C22H29BN4O6/c1-15(2)11-20(23(30)31)26-21(28)19(13-16-7-4-3-5-8-16)25-22(29)24-14-17-9-6-10-18(12-17)27(32)33/h3-10,12,15,19-20,30-31H,11,13-14H2,1-2H3,(H,26,28)(H2,24,25,29)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50259646

(CHEMBL4075225)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccc2ccccc2c1)B(O)O |r| Show InChI InChI=1S/C26H32BN3O4/c1-18(2)14-24(27(33)34)30-25(31)23(16-19-8-4-3-5-9-19)29-26(32)28-17-20-12-13-21-10-6-7-11-22(21)15-20/h3-13,15,18,23-24,33-34H,14,16-17H2,1-2H3,(H,30,31)(H2,28,29,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50259659

(CHEMBL4076838)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(c1)[N+]([O-])=O)B(O)O |r| Show InChI InChI=1S/C22H29BN4O6/c1-15(2)11-20(23(30)31)26-21(28)19(13-16-7-4-3-5-8-16)25-22(29)24-14-17-9-6-10-18(12-17)27(32)33/h3-10,12,15,19-20,30-31H,11,13-14H2,1-2H3,(H,26,28)(H2,24,25,29)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of 20S proteasome in human HL60 cells using Z-LRRaminoluciferin as substrate after 2 hrs by fluorescence analysis |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50259657

(CHEMBL4068221)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N(C)Cc1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-17(2)14-21(24(30)31)26-22(28)20(15-18-10-6-4-7-11-18)25-23(29)27(3)16-19-12-8-5-9-13-19/h4-13,17,20-21,30-31H,14-16H2,1-3H3,(H,25,29)(H,26,28)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of 20S proteasome in human HL60 cells using Z-LRRaminoluciferin as substrate after 2 hrs by fluorescence analysis |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259660
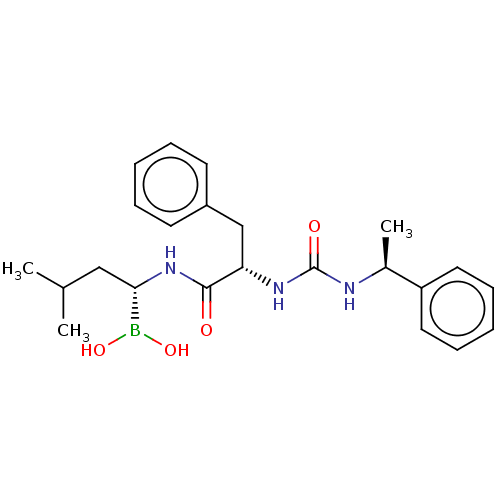
(CHEMBL4075298)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N[C@@H](C)c1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-16(2)14-21(24(30)31)27-22(28)20(15-18-10-6-4-7-11-18)26-23(29)25-17(3)19-12-8-5-9-13-19/h4-13,16-17,20-21,30-31H,14-15H2,1-3H3,(H,27,28)(H2,25,26,29)/t17-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259645

(CHEMBL4070336)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(c1)-c1ccccc1)B(O)O |r| Show InChI InChI=1S/C28H34BN3O4/c1-20(2)16-26(29(35)36)32-27(33)25(18-21-10-5-3-6-11-21)31-28(34)30-19-22-12-9-15-24(17-22)23-13-7-4-8-14-23/h3-15,17,20,25-26,35-36H,16,18-19H2,1-2H3,(H,32,33)(H2,30,31,34)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259649

(CHEMBL4077282)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCCc1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-17(2)15-21(24(30)31)27-22(28)20(16-19-11-7-4-8-12-19)26-23(29)25-14-13-18-9-5-3-6-10-18/h3-12,17,20-21,30-31H,13-16H2,1-2H3,(H,27,28)(H2,25,26,29)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of 20S proteasome in human HL60 cells using Z-nLPnLDaminoluciferin as substrate after 2 hrs by fluorescence analy... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259660

(CHEMBL4075298)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N[C@@H](C)c1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-16(2)14-21(24(30)31)27-22(28)20(15-18-10-6-4-7-11-18)26-23(29)25-17(3)19-12-8-5-9-13-19/h4-13,16-17,20-21,30-31H,14-15H2,1-3H3,(H,27,28)(H2,25,26,29)/t17-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of 20S proteasome in human HL60 cells using Z-nLPnLDaminoluciferin as substrate after 2 hrs by fluorescence analy... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50259642

(CHEMBL4077037)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(C)c1C)B(O)O |r| Show InChI InChI=1S/C24H34BN3O4/c1-16(2)13-22(25(31)32)28-23(29)21(14-19-10-6-5-7-11-19)27-24(30)26-15-20-12-8-9-17(3)18(20)4/h5-12,16,21-22,31-32H,13-15H2,1-4H3,(H,28,29)(H2,26,27,30)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of 20S proteasome in human HL60 cells using Z-LRRaminoluciferin as substrate after 2 hrs by fluorescence analysis |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50259647

(CHEMBL4100727)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1ccc(F)c(F)c1)B(O)O |r| Show InChI InChI=1S/C22H28BF2N3O4/c1-14(2)10-20(23(31)32)28-21(29)19(12-15-6-4-3-5-7-15)27-22(30)26-13-16-8-9-17(24)18(25)11-16/h3-9,11,14,19-20,31-32H,10,12-13H2,1-2H3,(H,28,29)(H2,26,27,30)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of 20S proteasome in human HL60 cells using Z-LRRaminoluciferin as substrate after 2 hrs by fluorescence analysis |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259644

(CHEMBL4091983)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cc(Cl)ccc1Cl)B(O)O |r| Show InChI InChI=1S/C22H28BCl2N3O4/c1-14(2)10-20(23(31)32)28-21(29)19(11-15-6-4-3-5-7-15)27-22(30)26-13-16-12-17(24)8-9-18(16)25/h3-9,12,14,19-20,31-32H,10-11,13H2,1-2H3,(H,28,29)(H2,26,27,30)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50259644

(CHEMBL4091983)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cc(Cl)ccc1Cl)B(O)O |r| Show InChI InChI=1S/C22H28BCl2N3O4/c1-14(2)10-20(23(31)32)28-21(29)19(11-15-6-4-3-5-7-15)27-22(30)26-13-16-12-17(24)8-9-18(16)25/h3-9,12,14,19-20,31-32H,10-11,13H2,1-2H3,(H,28,29)(H2,26,27,30)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of 20S proteasome in human HL60 cells using Z-LRRaminoluciferin as substrate after 2 hrs by fluorescence analysis |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50259655

(CHEMBL4080306)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N[C@H](C)c1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-16(2)14-21(24(30)31)27-22(28)20(15-18-10-6-4-7-11-18)26-23(29)25-17(3)19-12-8-5-9-13-19/h4-13,16-17,20-21,30-31H,14-15H2,1-3H3,(H,27,28)(H2,25,26,29)/t17-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of 20S proteasome in human HL60 cells using Z-LRRaminoluciferin as substrate after 2 hrs by fluorescence analysis |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50259648
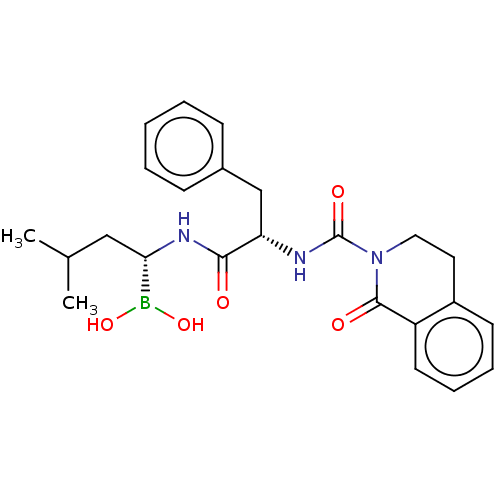
(CHEMBL4105585)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCc2ccccc2C1=O)B(O)O |r| Show InChI InChI=1S/C24H30BN3O5/c1-16(2)14-21(25(32)33)27-22(29)20(15-17-8-4-3-5-9-17)26-24(31)28-13-12-18-10-6-7-11-19(18)23(28)30/h3-11,16,20-21,32-33H,12-15H2,1-2H3,(H,26,31)(H,27,29)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of 20S proteasome in human HL60 cells using Suc-LLVYaminoluciferin as substrate after 2 hrs by fluorescence ... |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50259643

(CHEMBL4065491)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cnccn1)B(O)O |r| Show InChI InChI=1S/C20H28BN5O4/c1-14(2)10-18(21(29)30)26-19(27)17(11-15-6-4-3-5-7-15)25-20(28)24-13-16-12-22-8-9-23-16/h3-9,12,14,17-18,29-30H,10-11,13H2,1-2H3,(H,26,27)(H2,24,25,28)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Dissociation constant against galectin-3 using competitive fluorescence polarization |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50259649

(CHEMBL4077282)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCCc1ccccc1)B(O)O |r| Show InChI InChI=1S/C23H32BN3O4/c1-17(2)15-21(24(30)31)27-22(28)20(16-19-11-7-4-8-12-19)26-23(29)25-14-13-18-9-5-3-6-10-18/h3-12,17,20-21,30-31H,13-16H2,1-2H3,(H,27,28)(H2,25,26,29)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of 20S proteasome in human HL60 cells using Z-LRRaminoluciferin as substrate after 2 hrs by fluorescence analysis |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50259645

(CHEMBL4070336)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)NCc1cccc(c1)-c1ccccc1)B(O)O |r| Show InChI InChI=1S/C28H34BN3O4/c1-20(2)16-26(29(35)36)32-27(33)25(18-21-10-5-3-6-11-21)31-28(34)30-19-22-12-9-15-24(17-22)23-13-7-4-8-14-23/h3-15,17,20,25-26,35-36H,16,18-19H2,1-2H3,(H,32,33)(H2,30,31,34)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of 20S proteasome in human HL60 cells using Z-LRRaminoluciferin as substrate after 2 hrs by fluorescence analysis |
Eur J Med Chem 125: 925-939 (2017)
Article DOI: 10.1016/j.ejmech.2016.10.023
BindingDB Entry DOI: 10.7270/Q2348NTK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data