 Found 26 hits with Last Name = 'a m subbaiah' and Initial = 'm'
Found 26 hits with Last Name = 'a m subbaiah' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50523558

(CHEMBL4460401)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@@H](NC(=O)[C@H](N)C(C)C)C(C)C)C(C)(C)C |r| Show InChI InChI=1S/C48H70N8O9/c1-29(2)37(49)41(57)52-38(30(3)4)44(60)65-36(35(26-31-18-14-13-15-19-31)51-42(58)39(47(5,6)7)53-45(61)63-11)28-56(55-43(59)40(48(8,9)10)54-46(62)64-12)27-32-21-23-33(24-22-32)34-20-16-17-25-50-34/h13-25,29-30,35-40H,26-28,49H2,1-12H3,(H,51,58)(H,52,57)(H,53,61)(H,54,62)(H,55,59)/t35-,36-,37+,38-,39+,40+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50523561

(CHEMBL4437104)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)C(C)C)C(C)(C)C |r| Show InChI InChI=1S/C48H70N8O9/c1-29(2)37(49)41(57)52-38(30(3)4)44(60)65-36(35(26-31-18-14-13-15-19-31)51-42(58)39(47(5,6)7)53-45(61)63-11)28-56(55-43(59)40(48(8,9)10)54-46(62)64-12)27-32-21-23-33(24-22-32)34-20-16-17-25-50-34/h13-25,29-30,35-40H,26-28,49H2,1-12H3,(H,51,58)(H,52,57)(H,53,61)(H,54,62)(H,55,59)/t35-,36-,37-,38-,39+,40+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50523559
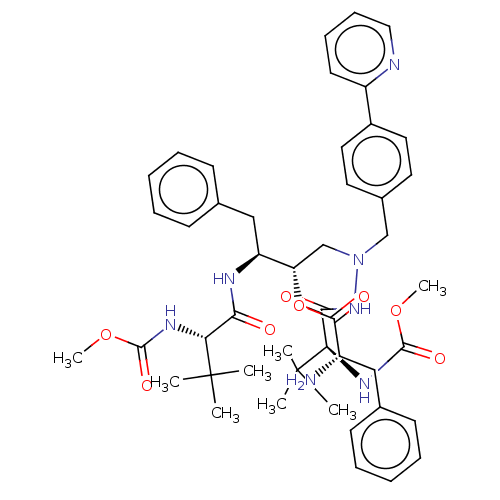
(CHEMBL4573907)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@@H](N)Cc1ccccc1)C(C)(C)C |r| Show InChI InChI=1S/C47H61N7O8/c1-46(2,3)39(51-44(58)60-7)41(55)50-37(28-32-19-13-10-14-20-32)38(62-43(57)35(48)27-31-17-11-9-12-18-31)30-54(53-42(56)40(47(4,5)6)52-45(59)61-8)29-33-22-24-34(25-23-33)36-21-15-16-26-49-36/h9-26,35,37-40H,27-30,48H2,1-8H3,(H,50,55)(H,51,58)(H,52,59)(H,53,56)/t35-,37-,38-,39+,40+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50523568

(CHEMBL4447493)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)CN)C(C)(C)C |r| Show InChI InChI=1S/C40H55N7O8/c1-39(2,3)33(44-37(51)53-7)35(49)43-30(22-26-14-10-9-11-15-26)31(55-32(48)23-41)25-47(46-36(50)34(40(4,5)6)45-38(52)54-8)24-27-17-19-28(20-18-27)29-16-12-13-21-42-29/h9-21,30-31,33-34H,22-25,41H2,1-8H3,(H,43,49)(H,44,51)(H,45,52)(H,46,50)/t30-,31-,33+,34+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50523567

(CHEMBL4522729)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@H](N)C(C)C)C(C)(C)C |r| Show InChI InChI=1S/C43H61N7O8/c1-27(2)34(44)39(53)58-33(32(24-28-16-12-11-13-17-28)46-37(51)35(42(3,4)5)47-40(54)56-9)26-50(49-38(52)36(43(6,7)8)48-41(55)57-10)25-29-19-21-30(22-20-29)31-18-14-15-23-45-31/h11-23,27,32-36H,24-26,44H2,1-10H3,(H,46,51)(H,47,54)(H,48,55)(H,49,52)/t32-,33-,34+,35+,36+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50523566

(CHEMBL4574382)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@H](N)Cc1ccccc1)C(C)(C)C |r| Show InChI InChI=1S/C47H61N7O8/c1-46(2,3)39(51-44(58)60-7)41(55)50-37(28-32-19-13-10-14-20-32)38(62-43(57)35(48)27-31-17-11-9-12-18-31)30-54(53-42(56)40(47(4,5)6)52-45(59)61-8)29-33-22-24-34(25-23-33)36-21-15-16-26-49-36/h9-26,35,37-40H,27-30,48H2,1-8H3,(H,50,55)(H,51,58)(H,52,59)(H,53,56)/t35-,37+,38+,39-,40-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50523560
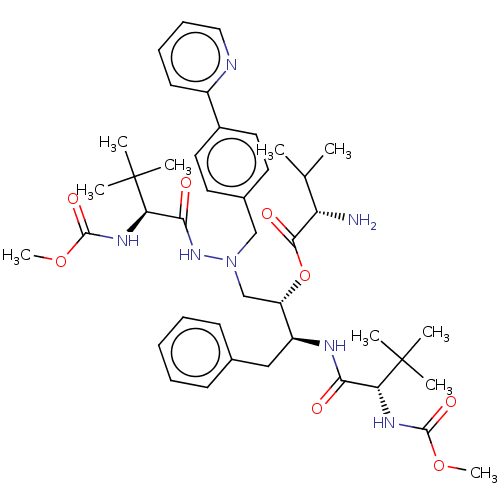
(CHEMBL4300203)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@@H](N)C(C)C)C(C)(C)C |r| Show InChI InChI=1S/C43H61N7O8/c1-27(2)34(44)39(53)58-33(32(24-28-16-12-11-13-17-28)46-37(51)35(42(3,4)5)47-40(54)56-9)26-50(49-38(52)36(43(6,7)8)48-41(55)57-10)25-29-19-21-30(22-20-29)31-18-14-15-23-45-31/h11-23,27,32-36H,24-26,44H2,1-10H3,(H,46,51)(H,47,54)(H,48,55)(H,49,52)/t32-,33-,34-,35+,36+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50523569

(CHEMBL4463796)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)CN(C)C)C(C)(C)C |r| Show InChI InChI=1S/C42H59N7O8/c1-41(2,3)35(45-39(53)55-9)37(51)44-32(24-28-16-12-11-13-17-28)33(57-34(50)27-48(7)8)26-49(47-38(52)36(42(4,5)6)46-40(54)56-10)25-29-19-21-30(22-20-29)31-18-14-15-23-43-31/h11-23,32-33,35-36H,24-27H2,1-10H3,(H,44,51)(H,45,53)(H,46,54)(H,47,52)/t32-,33-,35+,36+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50523562
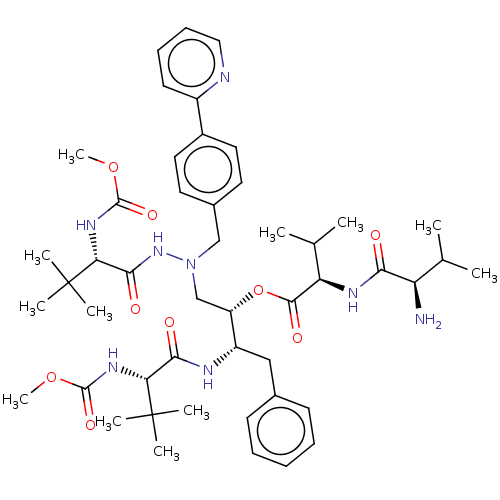
(CHEMBL4474072)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@H](NC(=O)[C@H](N)C(C)C)C(C)C)C(C)(C)C |r| Show InChI InChI=1S/C48H70N8O9/c1-29(2)37(49)41(57)52-38(30(3)4)44(60)65-36(35(26-31-18-14-13-15-19-31)51-42(58)39(47(5,6)7)53-45(61)63-11)28-56(55-43(59)40(48(8,9)10)54-46(62)64-12)27-32-21-23-33(24-22-32)34-20-16-17-25-50-34/h13-25,29-30,35-40H,26-28,49H2,1-12H3,(H,51,58)(H,52,57)(H,53,61)(H,54,62)(H,55,59)/t35-,36-,37+,38+,39+,40+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50523563

(CHEMBL4554438)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@H](NC(=O)[C@@H](N)C(C)C)C(C)C)C(C)(C)C |r| Show InChI InChI=1S/C48H70N8O9/c1-29(2)37(49)41(57)52-38(30(3)4)44(60)65-36(35(26-31-18-14-13-15-19-31)51-42(58)39(47(5,6)7)53-45(61)63-11)28-56(55-43(59)40(48(8,9)10)54-46(62)64-12)27-32-21-23-33(24-22-32)34-20-16-17-25-50-34/h13-25,29-30,35-40H,26-28,49H2,1-12H3,(H,51,58)(H,52,57)(H,53,61)(H,54,62)(H,55,59)/t35-,36-,37-,38+,39+,40+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50523564

(CHEMBL4525849)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@H](C)N)C(C)(C)C |r| Show InChI InChI=1S/C41H57N7O8/c1-26(42)37(51)56-32(31(23-27-15-11-10-12-16-27)44-35(49)33(40(2,3)4)45-38(52)54-8)25-48(47-36(50)34(41(5,6)7)46-39(53)55-9)24-28-18-20-29(21-19-28)30-17-13-14-22-43-30/h10-22,26,31-34H,23-25,42H2,1-9H3,(H,44,49)(H,45,52)(H,46,53)(H,47,50)/t26-,31-,32-,33+,34+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50523565
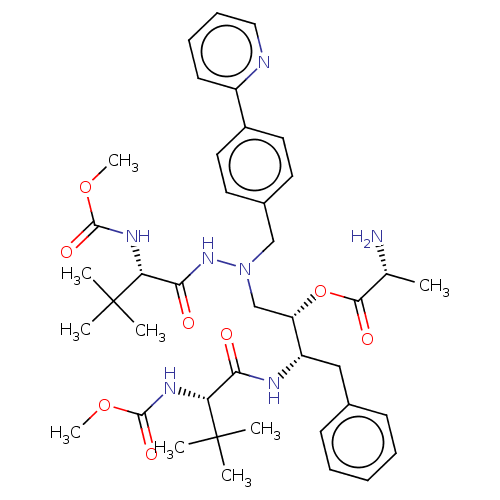
(CHEMBL4542773)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@@H](C)N)C(C)(C)C |r| Show InChI InChI=1S/C41H57N7O8/c1-26(42)37(51)56-32(31(23-27-15-11-10-12-16-27)44-35(49)33(40(2,3)4)45-38(52)54-8)25-48(47-36(50)34(41(5,6)7)46-39(53)55-9)24-28-18-20-29(21-19-28)30-17-13-14-22-43-30/h10-22,26,31-34H,23-25,42H2,1-9H3,(H,44,49)(H,45,52)(H,46,53)(H,47,50)/t26-,31+,32+,33-,34-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease activity |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Homo sapiens (Human)) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human UGT1A1 using bilirubin as substrate preincubated for 5 mins followed by substrate addition and measured after 40 mins... |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Homo sapiens (Human)) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00632
BindingDB Entry DOI: 10.7270/Q2SB49SR |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Homo sapiens (Human)) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00632
BindingDB Entry DOI: 10.7270/Q2SB49SR |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Homo sapiens (Human)) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of UGT1A1 in human liver microsomes using beta-estradiol as substrate preincubated for 5 mins followed by substrate addition and measured ... |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Homo sapiens (Human)) | BDBM50599144
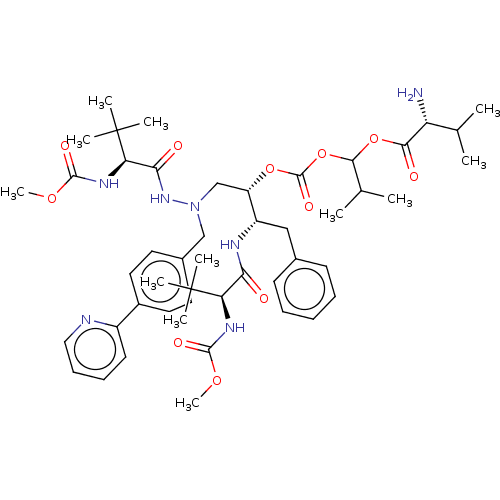
(CHEMBL5179012)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)OC(OC(=O)[C@H](N)C(C)C)C(C)C)C(C)(C)C |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00632
BindingDB Entry DOI: 10.7270/Q2SB49SR |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Homo sapiens (Human)) | BDBM50599144

(CHEMBL5179012)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)OC(OC(=O)[C@H](N)C(C)C)C(C)C)C(C)(C)C |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00632
BindingDB Entry DOI: 10.7270/Q2SB49SR |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Homo sapiens (Human)) | BDBM50523559

(CHEMBL4573907)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@@H](N)Cc1ccccc1)C(C)(C)C |r| Show InChI InChI=1S/C47H61N7O8/c1-46(2,3)39(51-44(58)60-7)41(55)50-37(28-32-19-13-10-14-20-32)38(62-43(57)35(48)27-31-17-11-9-12-18-31)30-54(53-42(56)40(47(4,5)6)52-45(59)61-8)29-33-22-24-34(25-23-33)36-21-15-16-26-49-36/h9-26,35,37-40H,27-30,48H2,1-8H3,(H,50,55)(H,51,58)(H,52,59)(H,53,56)/t35-,37-,38-,39+,40+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of UGT1A1 in human liver microsomes using beta-estradiol as substrate preincubated for 5 mins followed by substrate addition and measured ... |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Homo sapiens (Human)) | BDBM50523559

(CHEMBL4573907)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@@H](N)Cc1ccccc1)C(C)(C)C |r| Show InChI InChI=1S/C47H61N7O8/c1-46(2,3)39(51-44(58)60-7)41(55)50-37(28-32-19-13-10-14-20-32)38(62-43(57)35(48)27-31-17-11-9-12-18-31)30-54(53-42(56)40(47(4,5)6)52-45(59)61-8)29-33-22-24-34(25-23-33)36-21-15-16-26-49-36/h9-26,35,37-40H,27-30,48H2,1-8H3,(H,50,55)(H,51,58)(H,52,59)(H,53,56)/t35-,37-,38-,39+,40+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human UGT1A1 using bilirubin as substrate preincubated for 5 mins followed by substrate addition and measured after 40 mins... |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Rattus norvegicus) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of UGT1A1 in rat liver microsomes using beta-estradiol as substrate preincubated for 5 mins followed by substrate addition and measured af... |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Rattus norvegicus) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat UGT1A1 using beta-estradiol as substrate preincubated for 5 mins followed by substrate addition and measured after 40 m... |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50599144

(CHEMBL5179012)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)OC(OC(=O)[C@H](N)C(C)C)C(C)C)C(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00632
BindingDB Entry DOI: 10.7270/Q2SB49SR |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Rattus norvegicus) | BDBM50523559

(CHEMBL4573907)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@@H](N)Cc1ccccc1)C(C)(C)C |r| Show InChI InChI=1S/C47H61N7O8/c1-46(2,3)39(51-44(58)60-7)41(55)50-37(28-32-19-13-10-14-20-32)38(62-43(57)35(48)27-31-17-11-9-12-18-31)30-54(53-42(56)40(47(4,5)6)52-45(59)61-8)29-33-22-24-34(25-23-33)36-21-15-16-26-49-36/h9-26,35,37-40H,27-30,48H2,1-8H3,(H,50,55)(H,51,58)(H,52,59)(H,53,56)/t35-,37-,38-,39+,40+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat UGT1A1 using beta-estradiol as substrate preincubated for 5 mins followed by substrate addition and measured after 40 m... |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 1A1
(Rattus norvegicus) | BDBM50523559

(CHEMBL4573907)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)OC(=O)[C@@H](N)Cc1ccccc1)C(C)(C)C |r| Show InChI InChI=1S/C47H61N7O8/c1-46(2,3)39(51-44(58)60-7)41(55)50-37(28-32-19-13-10-14-20-32)38(62-43(57)35(48)27-31-17-11-9-12-18-31)30-54(53-42(56)40(47(4,5)6)52-45(59)61-8)29-33-22-24-34(25-23-33)36-21-15-16-26-49-36/h9-26,35,37-40H,27-30,48H2,1-8H3,(H,50,55)(H,51,58)(H,52,59)(H,53,56)/t35-,37-,38-,39+,40+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of UGT1A1 in rat liver microsomes using beta-estradiol as substrate preincubated for 5 mins followed by substrate addition and measured af... |
J Med Chem 62: 3553-3574 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00002
BindingDB Entry DOI: 10.7270/Q2BP066S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data