

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of recombinant full-length GST-tagged human B-RAF V600E mutant (417 to 766 residues) expressed in Baculovirus infected Sf9 cells using N-t... | Eur J Med Chem 150: 567-578 (2018) Article DOI: 10.1016/j.ejmech.2018.03.001 BindingDB Entry DOI: 10.7270/Q26D5WNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of His-6 tagged recombinant EGFR cytoplasmic domain (645 to 1186 residues) (unknown origin) expressed in Baculovirus infected Sf9 cells by... | Eur J Med Chem 150: 567-578 (2018) Article DOI: 10.1016/j.ejmech.2018.03.001 BindingDB Entry DOI: 10.7270/Q26D5WNS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM222457 ((E)-4-[2-(4-Chlorophenyl)-5-oxo-4-(3,4,5-trimethox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM222461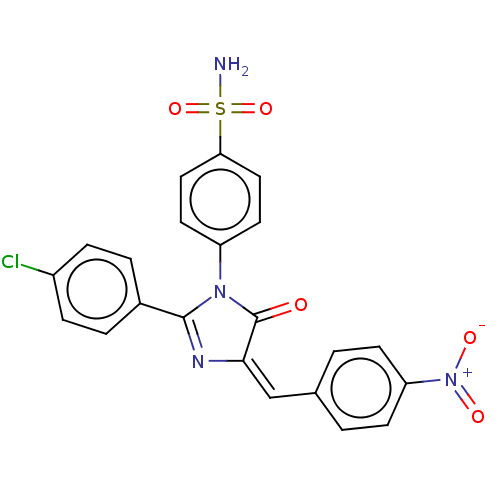 ((E)-4-[2-(4-Chloro-phenyl)-4-(4-nitrobenzylidene)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM222460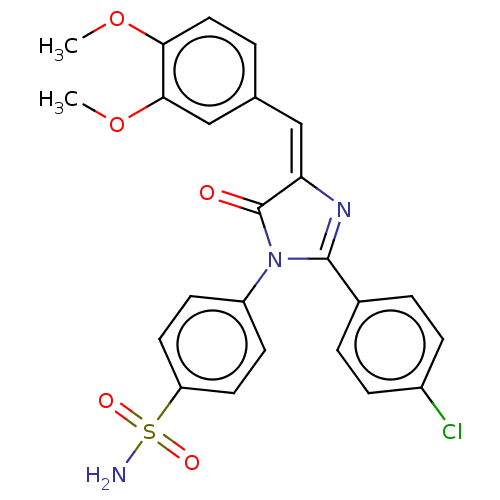 ((E)-4-[2-(4-Chlorophenyl)-4-(3,4-dimethoxybenzylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM222458 ((E)-4-[2-(4-Chlorophenyl)-5-oxo-4-(4-trifluorometh...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM222462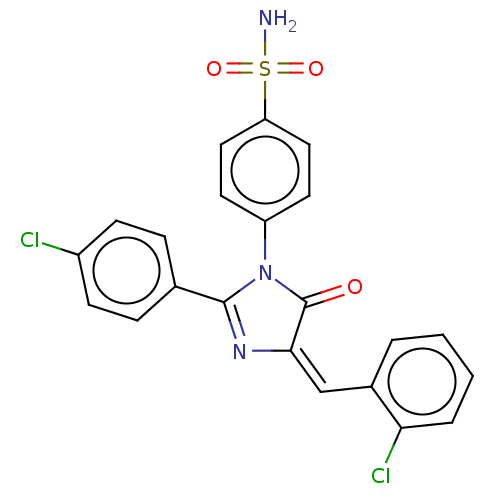 ((E)-4-[4-(2-Chlorobenzylidene)-2-(4-chlorophenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM222459 ((E)-4-[2-(4-Chlorophenyl)-4-(2,3-dimethoxybenzylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM222463 ((E)-4-[4-(4-Chlorobenzylidene)-2-(4-chlorophenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50462234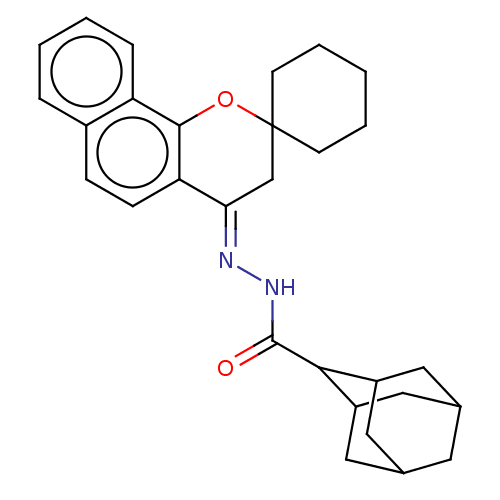 (CHEMBL4241486) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of His-6 tagged recombinant EGFR cytoplasmic domain (645 to 1186 residues) (unknown origin) expressed in Baculovirus infected Sf9 cells by... | Eur J Med Chem 150: 567-578 (2018) Article DOI: 10.1016/j.ejmech.2018.03.001 BindingDB Entry DOI: 10.7270/Q26D5WNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50462233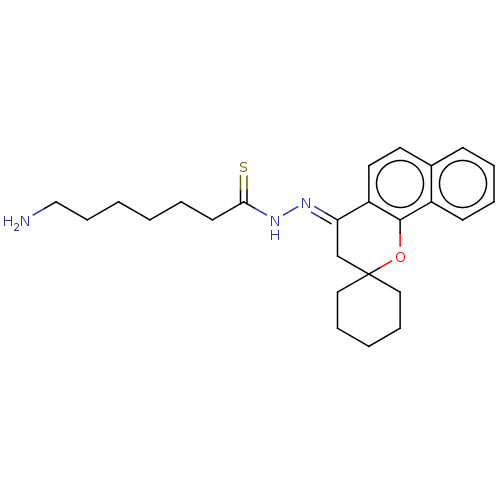 (CHEMBL4244030) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of His-6 tagged recombinant EGFR cytoplasmic domain (645 to 1186 residues) (unknown origin) expressed in Baculovirus infected Sf9 cells by... | Eur J Med Chem 150: 567-578 (2018) Article DOI: 10.1016/j.ejmech.2018.03.001 BindingDB Entry DOI: 10.7270/Q26D5WNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50462232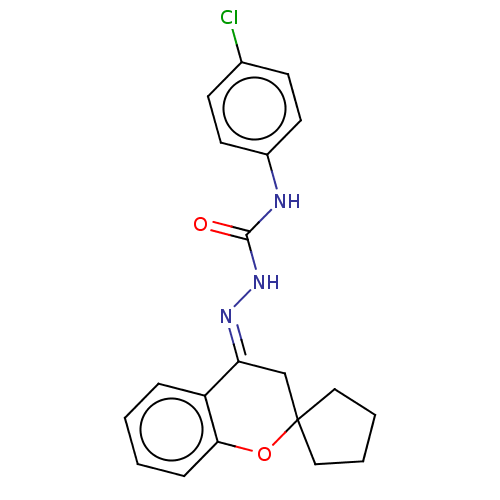 (CHEMBL4241867) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of His-6 tagged recombinant EGFR cytoplasmic domain (645 to 1186 residues) (unknown origin) expressed in Baculovirus infected Sf9 cells by... | Eur J Med Chem 150: 567-578 (2018) Article DOI: 10.1016/j.ejmech.2018.03.001 BindingDB Entry DOI: 10.7270/Q26D5WNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM222464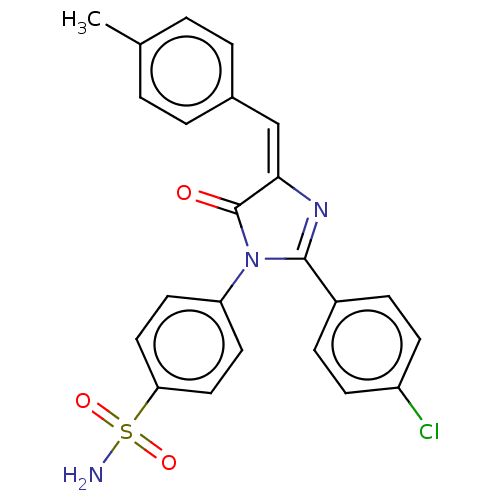 ((E)-4-[2-(4-Chlorophenyl)-4-(4-methylbenzylidene)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50462234 (CHEMBL4241486) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of recombinant full-length GST-tagged human B-RAF V600E mutant (417 to 766 residues) expressed in Baculovirus infected Sf9 cells using N-t... | Eur J Med Chem 150: 567-578 (2018) Article DOI: 10.1016/j.ejmech.2018.03.001 BindingDB Entry DOI: 10.7270/Q26D5WNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50462233 (CHEMBL4244030) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of recombinant full-length GST-tagged human B-RAF V600E mutant (417 to 766 residues) expressed in Baculovirus infected Sf9 cells using N-t... | Eur J Med Chem 150: 567-578 (2018) Article DOI: 10.1016/j.ejmech.2018.03.001 BindingDB Entry DOI: 10.7270/Q26D5WNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50462232 (CHEMBL4241867) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University Curated by ChEMBL | Assay Description Inhibition of recombinant full-length GST-tagged human B-RAF V600E mutant (417 to 766 residues) expressed in Baculovirus infected Sf9 cells using N-t... | Eur J Med Chem 150: 567-578 (2018) Article DOI: 10.1016/j.ejmech.2018.03.001 BindingDB Entry DOI: 10.7270/Q26D5WNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM222460 ((E)-4-[2-(4-Chlorophenyl)-4-(3,4-dimethoxybenzylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM222461 ((E)-4-[2-(4-Chloro-phenyl)-4-(4-nitrobenzylidene)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM222457 ((E)-4-[2-(4-Chlorophenyl)-5-oxo-4-(3,4,5-trimethox...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM222459 ((E)-4-[2-(4-Chlorophenyl)-4-(2,3-dimethoxybenzylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM222458 ((E)-4-[2-(4-Chlorophenyl)-5-oxo-4-(4-trifluorometh...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM222462 ((E)-4-[4-(2-Chlorobenzylidene)-2-(4-chlorophenyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM222463 ((E)-4-[4-(4-Chlorobenzylidene)-2-(4-chlorophenyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM222464 ((E)-4-[2-(4-Chlorophenyl)-4-(4-methylbenzylidene)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University; Ibn Sina National College for Medical Studies | Assay Description The ability of the test compounds 10a-h listed in Table 1 to inhibit ovine COX-1 and human recombinant COX-2 (IC50 value, mM) was determined using an... | Bioorg Chem 72: 123-129 (2017) Article DOI: 10.1016/j.bioorg.2017.04.002 BindingDB Entry DOI: 10.7270/Q2MS3RMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||