

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50121716 ((3-methyl-4-(3-(pyridin-3-ylmethylamino)propoxy)be...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220561 (CHEMBL173370) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220562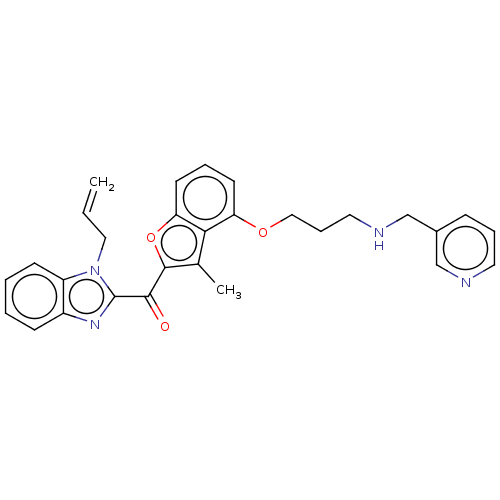 (CHEMBL171506) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220565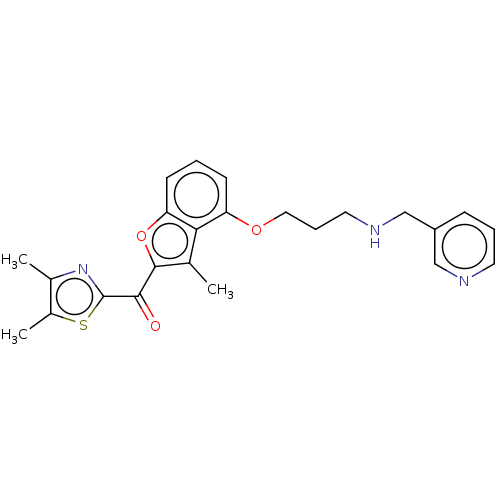 (CHEMBL170524) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50121720 ((1-METHYL-1H-IMIDAZOL-2-YL)-(3-METHYL-4-{3-[(PYRID...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220569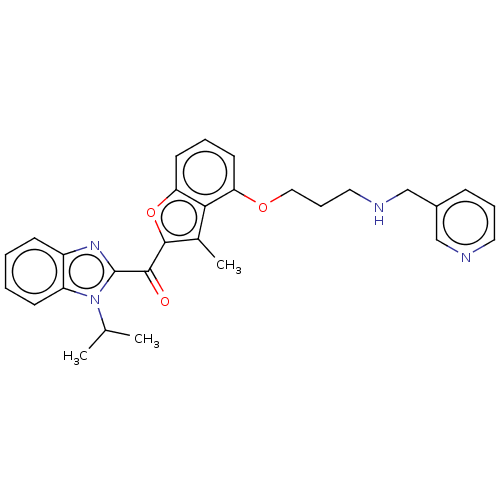 (CHEMBL422896) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220559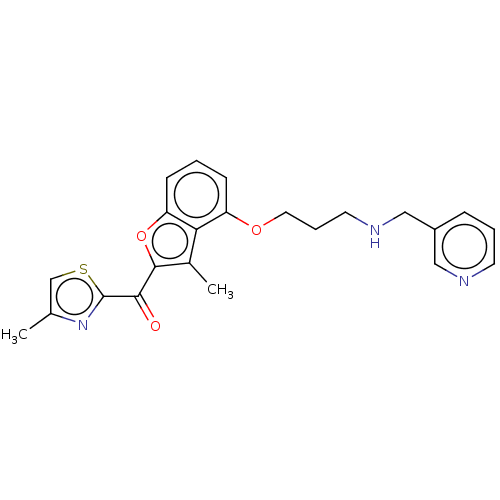 (CHEMBL422746) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220571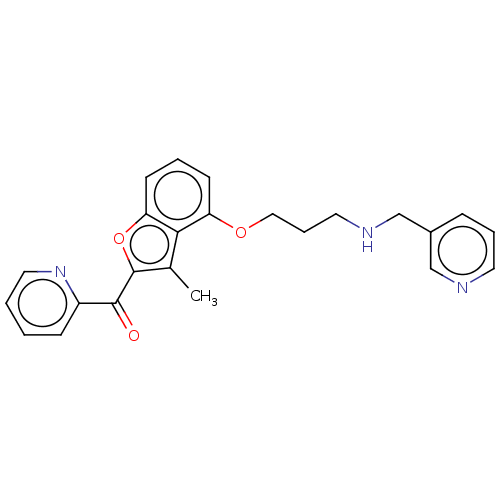 (CHEMBL355250) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062251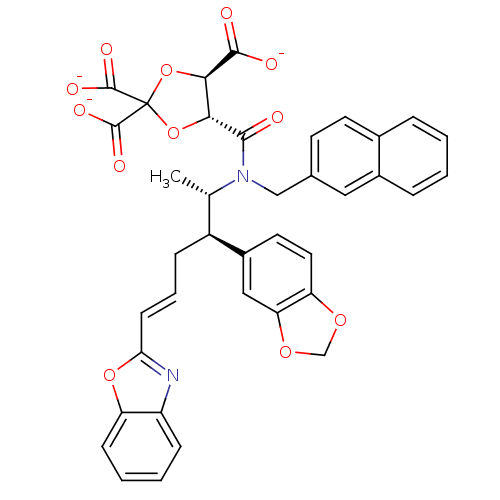 (CHEMBL285263 | trisodium 5-[2-benzo[d][1,3]dioxol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062250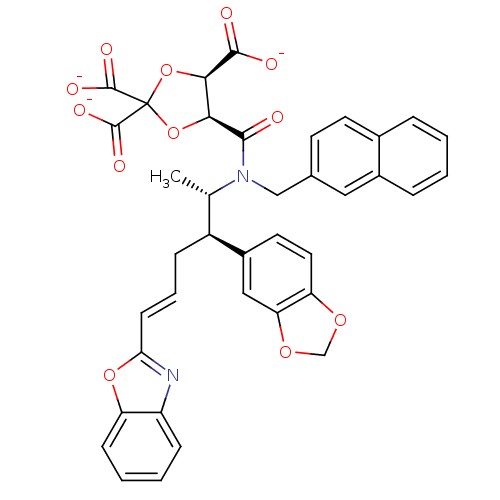 (CHEMBL36407 | trisodium 5-[2-benzo[d][1,3]dioxol-5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249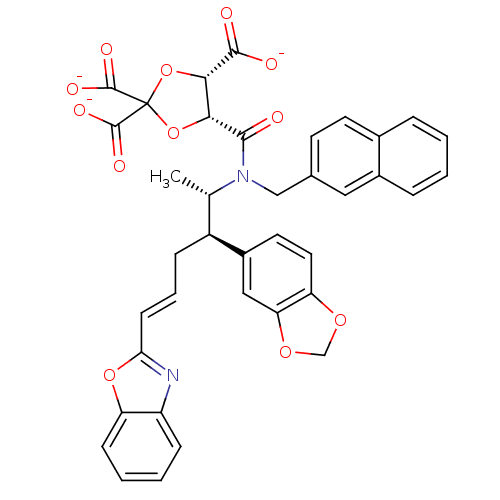 (CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50109882 (3-(3-methyl-2-((2,3,4-trifluorophenoxy)methyl)benz...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity against Candida albicans Nmt (CaNmt) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249 (CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase in competitive manner with respect to FPP (farnesyl diphosphate) at 0.6 microM FPP and 0.36 microM Ras peptide | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062252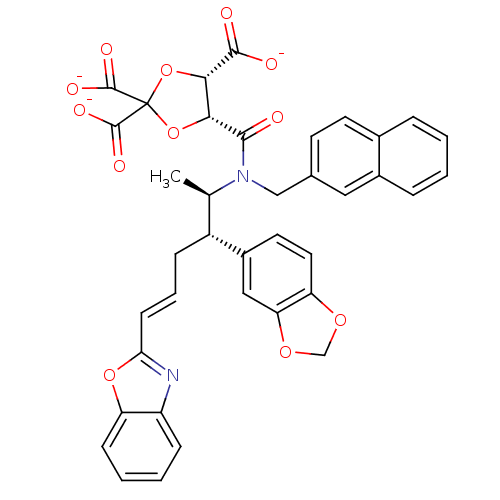 (CHEMBL36339 | trisodium 5-[2-benzo[d][1,3]dioxol-5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249 (CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase in competitive manner with respect to FPP (farnesyl diphosphate) at 0.6 microM FPP and 3.6 microM Ras peptide. | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062248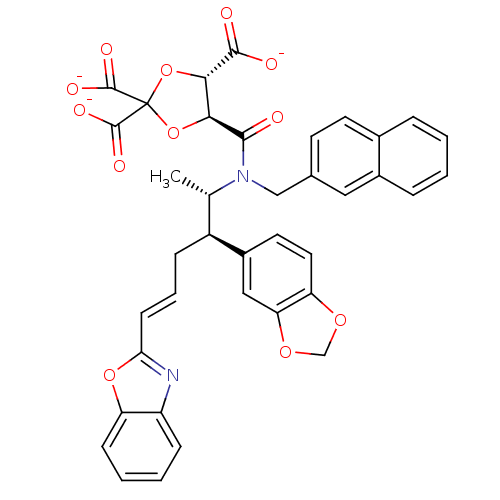 (CHEMBL36515 | trisodium 5-[2-benzo[d][1,3]dioxol-5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062256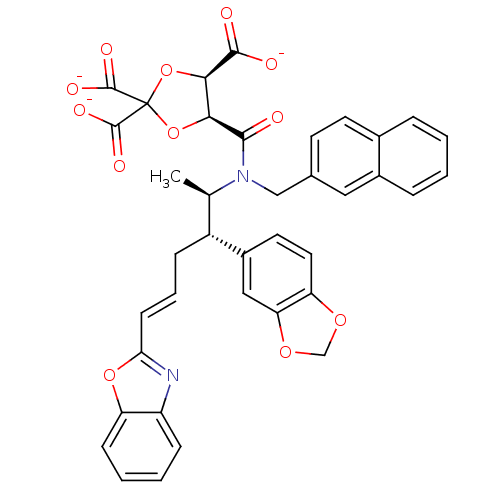 (CHEMBL284073 | trisodium 5-[2-benzo[d][1,3]dioxol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062258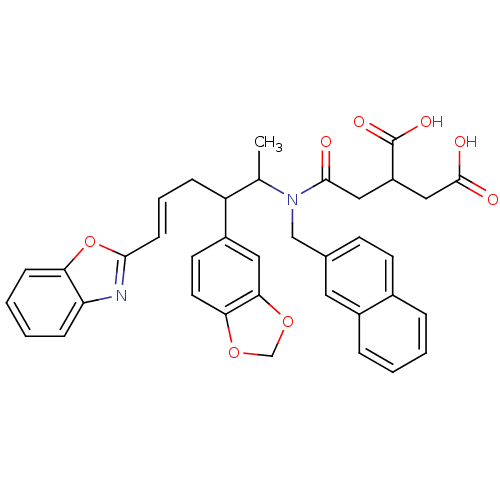 (2-{[((E)-2-Benzo[1,3]dioxol-5-yl-5-benzooxazol-2-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062257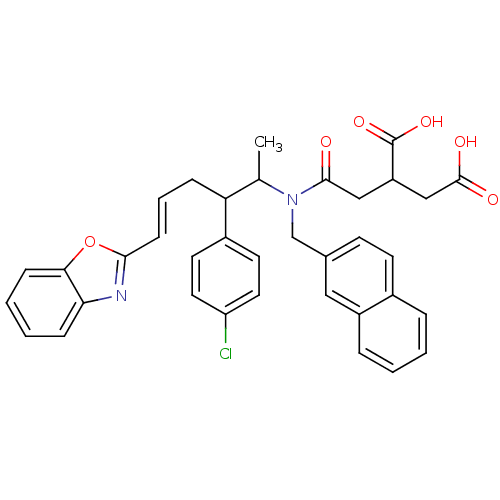 (2-({[(E)-5-Benzooxazol-2-yl-2-(4-chloro-phenyl)-1-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220564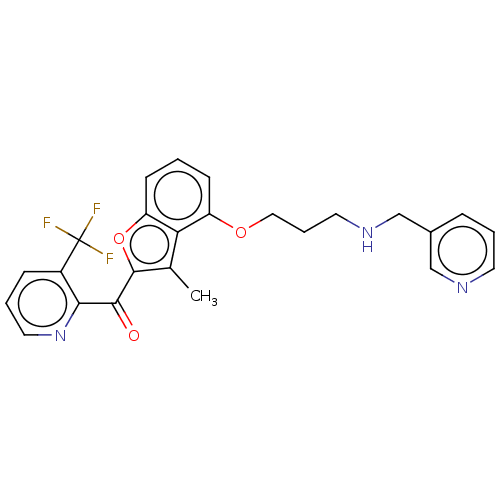 (CHEMBL353541) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062249 (CHEMBL285434 | trisodium 5-[2-benzo[d][1,3]dioxol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of FTase in competitive manner with respect to FPP (farnesyl diphosphate) at 6.0 microM FPP and 0.36 microM Ras peptide. | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220566 (CHEMBL172471) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062253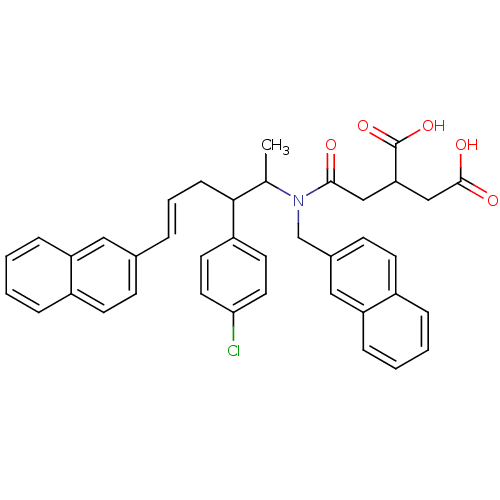 (2-({[(E)-2-(4-Chloro-phenyl)-1-methyl-5-naphthalen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220563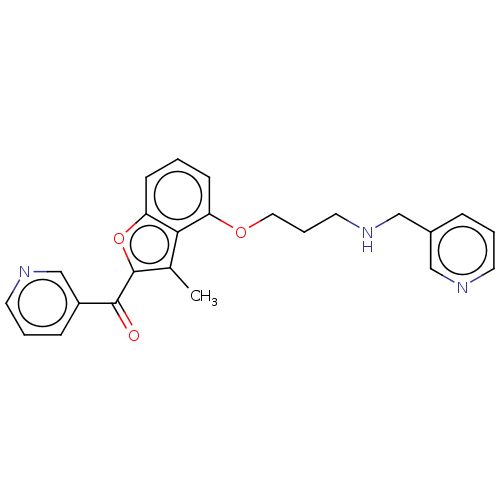 (CHEMBL353249) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220560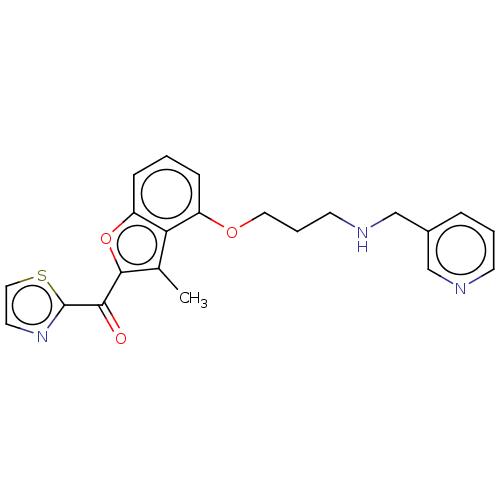 (CHEMBL173962) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6655 (3-(9-oxo-9H-fluoren-4-yl)-1-pyridin-2-ylurea | Dia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | 30 |
Banyu Tsukuba Research Institute | Assay Description In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d... | J Med Chem 44: 4615-27 (2001) Article DOI: 10.1021/jm0103256 BindingDB Entry DOI: 10.7270/Q2736P3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220569 (CHEMBL422896) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aspergillus fumigatus Nmt (AfNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6656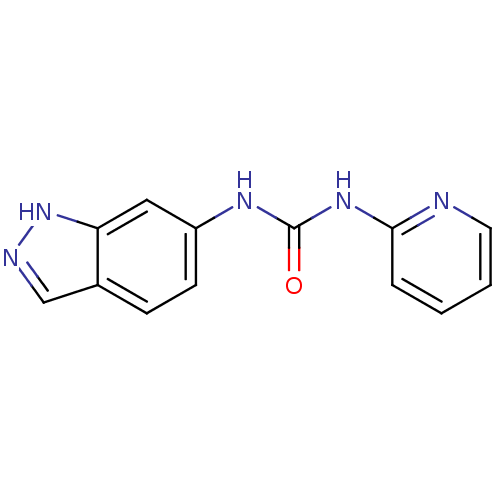 (3-1H-indazol-6-yl-1-pyridin-2-ylurea | Diarylurea ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | 30 |
Banyu Tsukuba Research Institute | Assay Description In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d... | J Med Chem 44: 4615-27 (2001) Article DOI: 10.1021/jm0103256 BindingDB Entry DOI: 10.7270/Q2736P3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220570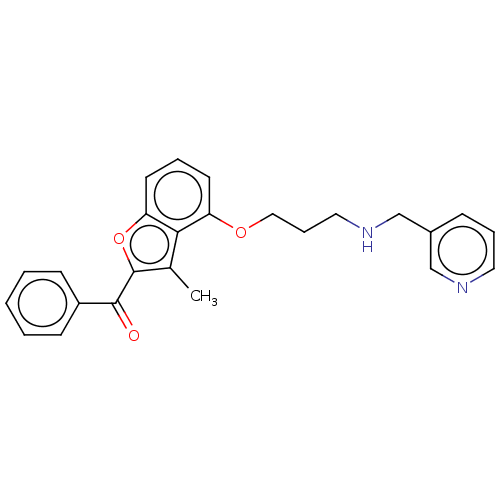 (CHEMBL172455) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220568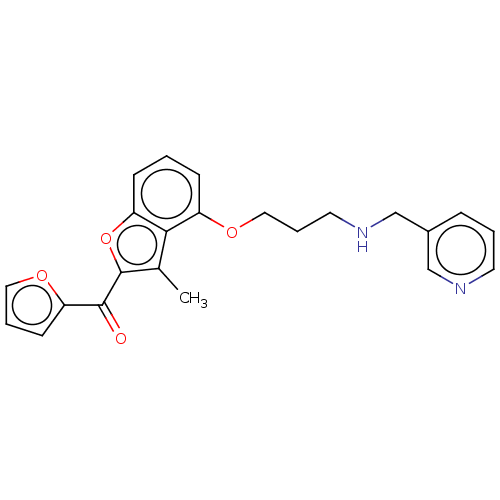 (CHEMBL368134) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220567 (CHEMBL355170) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory activity against Candida albicans Nmt (CaNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6657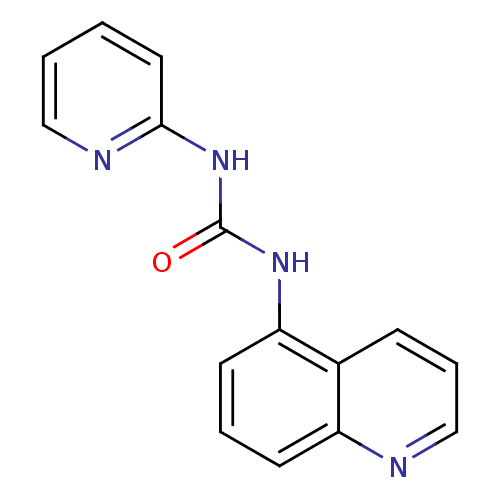 (3-pyridin-2-yl-1-quinolin-5-ylurea | Diarylurea de...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | 30 |
Banyu Tsukuba Research Institute | Assay Description In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d... | J Med Chem 44: 4615-27 (2001) Article DOI: 10.1021/jm0103256 BindingDB Entry DOI: 10.7270/Q2736P3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062255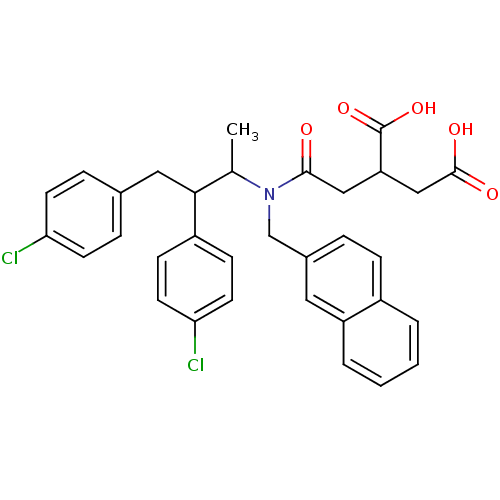 (2-({[2,3-Bis-(4-chloro-phenyl)-1-methyl-propyl]-na...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6658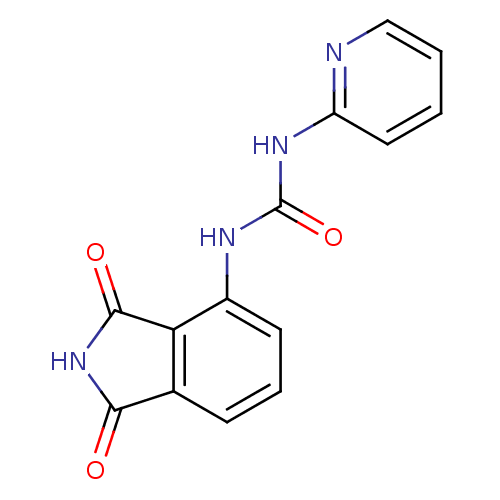 (3-(1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl)-1-pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | 30 |
Banyu Tsukuba Research Institute | Assay Description In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d... | J Med Chem 44: 4615-27 (2001) Article DOI: 10.1021/jm0103256 BindingDB Entry DOI: 10.7270/Q2736P3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50121716 ((3-methyl-4-(3-(pyridin-3-ylmethylamino)propoxy)be...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against aspergillus fumigatus Nmt (AfNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220565 (CHEMBL170524) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aspergillus fumigatus Nmt (AfNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220562 (CHEMBL171506) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aspergillus fumigatus Nmt (AfNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6654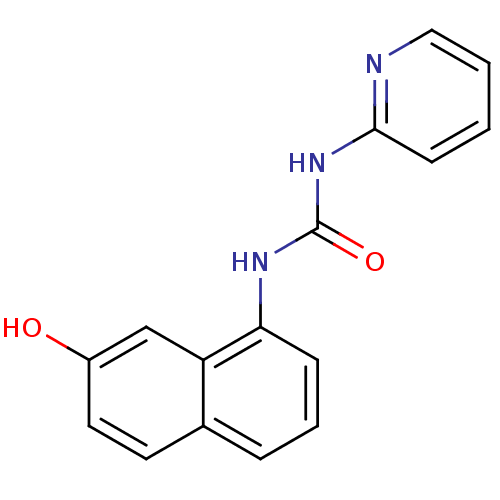 (3-(7-hydroxynaphthalen-1-yl)-1-pyridin-2-ylurea | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | 30 |
Banyu Tsukuba Research Institute | Assay Description In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d... | J Med Chem 44: 4615-27 (2001) Article DOI: 10.1021/jm0103256 BindingDB Entry DOI: 10.7270/Q2736P3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50062254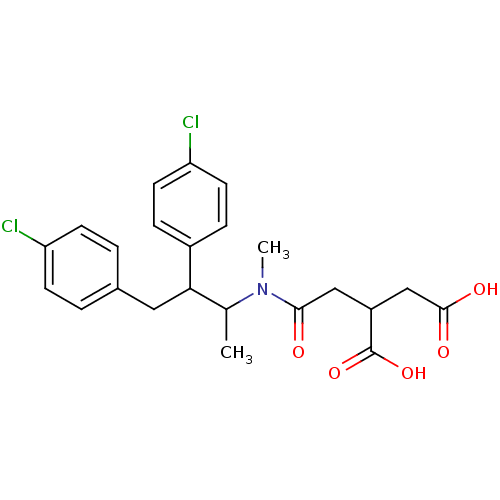 (2-({[2,3-Bis-(4-chloro-phenyl)-1-methyl-propyl]-me...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of Farnesyltransferase from rat brain | J Med Chem 41: 143-7 (1998) Article DOI: 10.1021/jm970540f BindingDB Entry DOI: 10.7270/Q23B5Z82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220564 (CHEMBL353541) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aspergillus fumigatus Nmt (AfNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220561 (CHEMBL173370) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aspergillus fumigatus Nmt (AfNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6641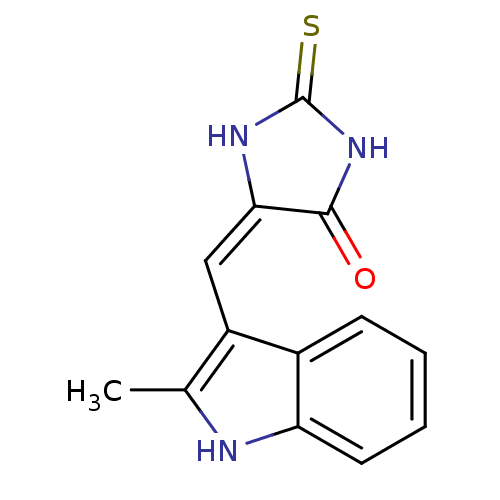 ((5E)-5-[(2-methyl-1H-indol-3-yl)methylidene]-2-sul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Banyu Tsukuba Research Institute | Assay Description In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d... | J Med Chem 44: 4615-27 (2001) Article DOI: 10.1021/jm0103256 BindingDB Entry DOI: 10.7270/Q2736P3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6645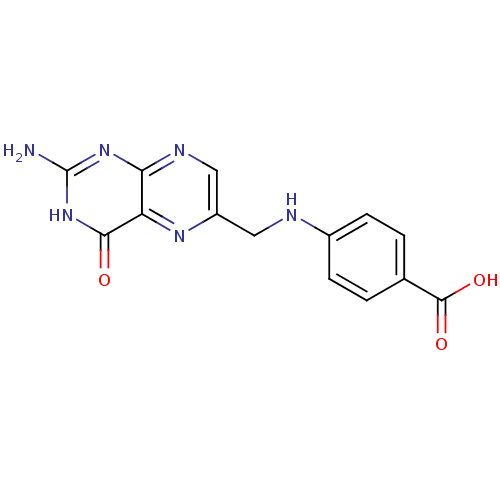 (4-{[(2-amino-4-hydroxypteridin-6-yl)methyl]amino}b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Banyu Tsukuba Research Institute | Assay Description In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d... | J Med Chem 44: 4615-27 (2001) Article DOI: 10.1021/jm0103256 BindingDB Entry DOI: 10.7270/Q2736P3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220559 (CHEMBL422746) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aspergillus fumigatus Nmt (AfNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6649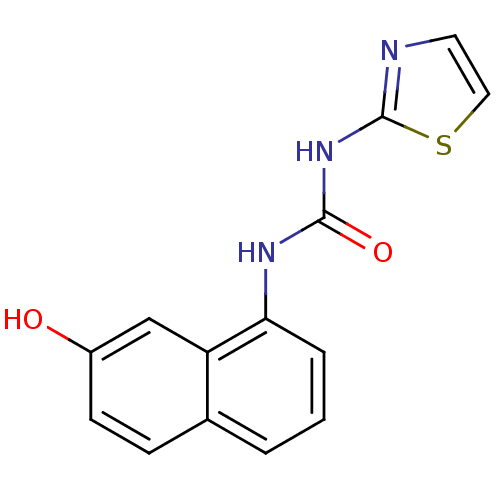 (1-(7-hydroxynaphthalen-1-yl)-3-1,3-thiazol-2-ylure...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
Banyu Tsukuba Research Institute | Assay Description In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d... | J Med Chem 44: 4615-27 (2001) Article DOI: 10.1021/jm0103256 BindingDB Entry DOI: 10.7270/Q2736P3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50220560 (CHEMBL173962) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Nmt (HsNmt) using 0.5 uM peptide GNAASAR-R-NH2 and 0.5 uM myristoyl-CoA | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1/2 (Homo sapiens (Human)) | BDBM50121720 ((1-METHYL-1H-IMIDAZOL-2-YL)-(3-METHYL-4-{3-[(PYRID...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aspergillus fumigatus Nmt (AfNmt) using substrate peptide GLTISKLFR-R-NH2 (0.5 uM) and myristoyl-CoA (0.5 uM) | Bioorg Med Chem Lett 13: 87-91 (2003) BindingDB Entry DOI: 10.7270/Q25D8V2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6643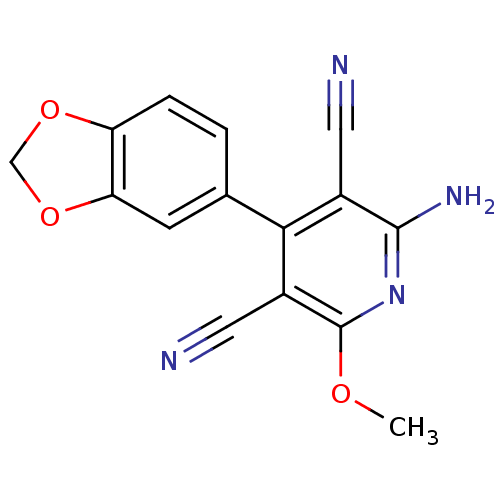 (2-amino-4-(2H-1,3-benzodioxol-5-yl)-6-methoxypyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Banyu Tsukuba Research Institute | Assay Description In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d... | J Med Chem 44: 4615-27 (2001) Article DOI: 10.1021/jm0103256 BindingDB Entry DOI: 10.7270/Q2736P3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6636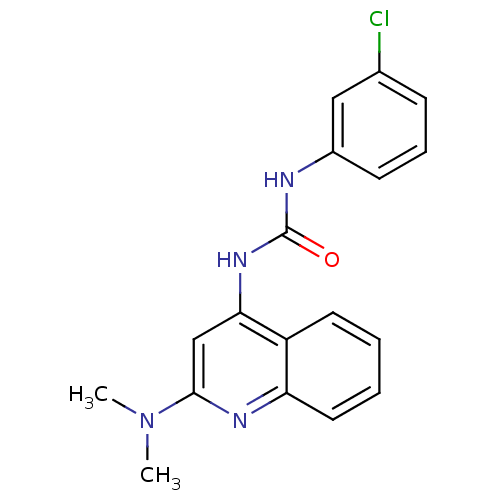 (3-(3-chlorophenyl)-1-[2-(dimethylamino)quinolin-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Banyu Tsukuba Research Institute | Assay Description In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d... | J Med Chem 44: 4615-27 (2001) Article DOI: 10.1021/jm0103256 BindingDB Entry DOI: 10.7270/Q2736P3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C] (Homo sapiens (Human)) | BDBM6638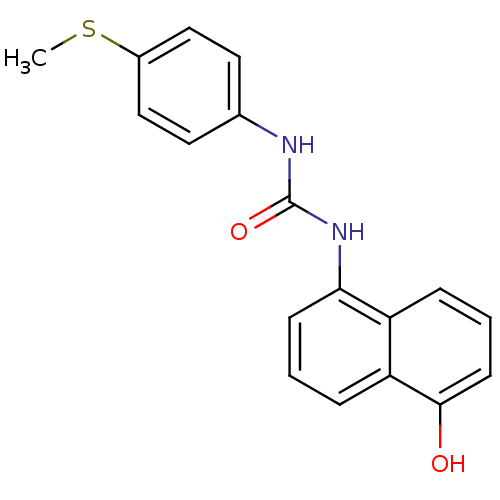 (1-(5-hydroxynaphthalen-1-yl)-3-[4-(methylsulfanyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Banyu Tsukuba Research Institute | Assay Description In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d... | J Med Chem 44: 4615-27 (2001) Article DOI: 10.1021/jm0103256 BindingDB Entry DOI: 10.7270/Q2736P3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |