

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of SK1 (unknown origin) using 5 uM of sphingosine as substrate | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50343835 ((S)-1-(4-(4-(3-(2-Cyclohexylethyl)phenyl)oxazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of SK1 (unknown origin) | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50139650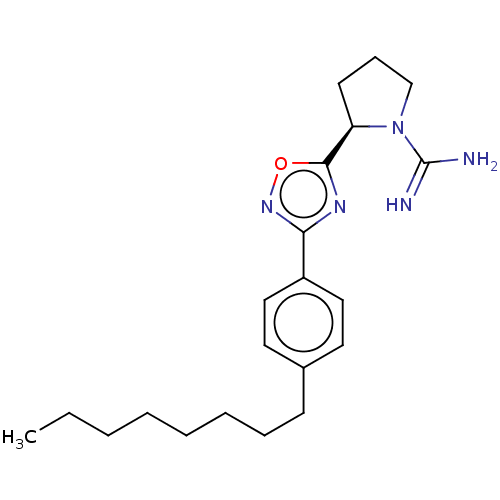 (CHEMBL3546834 | US9688668, 50) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of recombinant SK2 (unknown origin) expressed in Sf9 cells assessed as [33P]S1P formation using D-erythro sphingosine as substrate and gam... | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50343835 ((S)-1-(4-(4-(3-(2-Cyclohexylethyl)phenyl)oxazol-2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of SK2 (unknown origin) | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50017016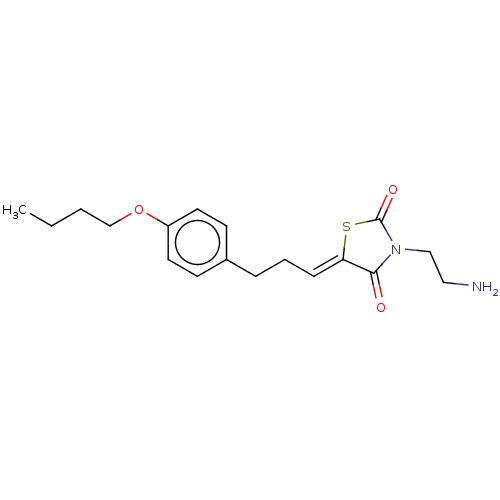 (CHEMBL3287036) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of recombinant SK2 (unknown origin) using sphingosine as substrate and gamma[32P]ATP by Lineweaver-Burk plot analysis | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50139649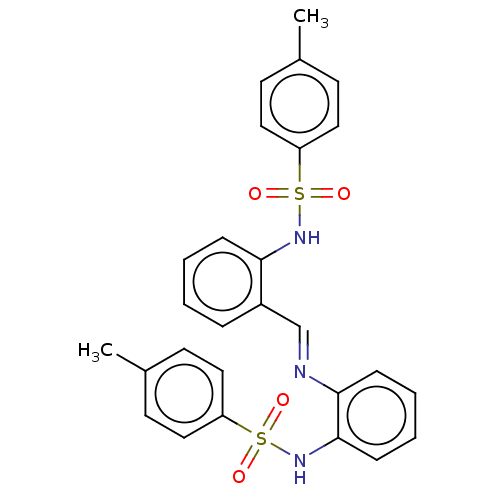 (CHEMBL3764617) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human SK2 using D-erythro sphingosine as substrate and gamma[33P]ATP by Lineweaver-Burk plot analysis | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50312869 (4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of SK2 (unknown origin) | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50393642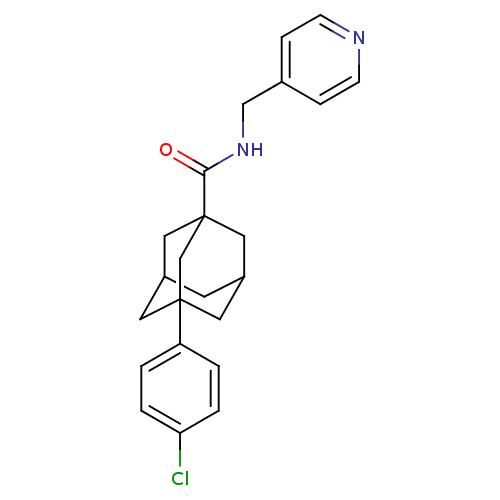 (CHEMBL2158685) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of SK2 (unknown origin) | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50139650 (CHEMBL3546834 | US9688668, 50) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of recombinant SK1 (unknown origin) expressed in Sf9 cells assessed as [33P]S1P formation using D-erythro sphingosine as substrate and gam... | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50312869 (4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of SK1 (unknown origin) | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50139649 (CHEMBL3764617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human SK1 using D-erythro sphingosine as substrate and gamma[33P]ATP by Lineweaver-Burk plot analysis | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of SK1 (unknown origin) using 3 uM of sphingosine as substrate | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50139648 (CHEMBL3763321) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of SK1 (unknown origin) | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of recombinant human SK2 assessed as production of [32P] S1P using 10 uM sphingosine as substrate by TLC method in presence of 100 uM [gam... | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50139648 (CHEMBL3763321) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of SK2 (unknown origin) | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 387 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human SK1 assessed as production of [32P]-S1P using 10 uM sphingosine as substrate by TLC method in presence of ... | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50139652 (CHEMBL3764032) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of recombinant human SK2 assessed as production of [32P] S1P using 10 uM sphingosine as substrate by TLC method in presence of 100 uM [gam... | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50139651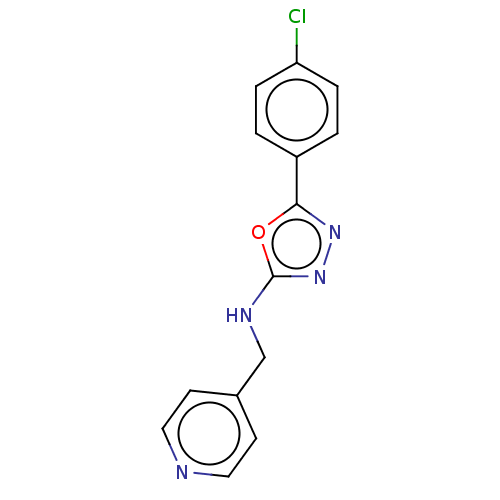 (CHEMBL3763496) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human SK1 assessed as production of [32P]-S1P using 10 uM sphingosine as substrate by TLC method in presence of ... | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50139651 (CHEMBL3763496) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of recombinant human SK2 assessed as production of [32P] S1P using 10 uM sphingosine as substrate by TLC method in presence of 100 uM [gam... | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of SK2 (unknown origin) | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of SK1 (unknown origin) | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50139652 (CHEMBL3764032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human SK1 assessed as production of [32P]-S1P using 10 uM sphingosine as substrate by TLC method in presence of ... | J Med Chem 59: 965-84 (2016) Article DOI: 10.1021/acs.jmedchem.5b01439 BindingDB Entry DOI: 10.7270/Q2WW7KJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304462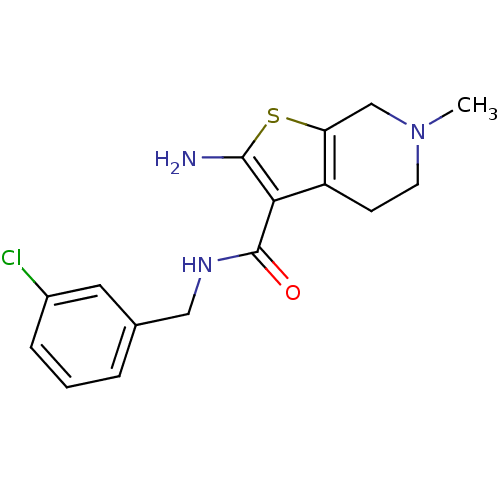 (2-Amino-N-(3-chlorobenzyl)-6-methyl-4,5,6,7-tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304463 (6-tert-Butyl 3-ethyl 2-amino-4,5-dihydrothieno[2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304464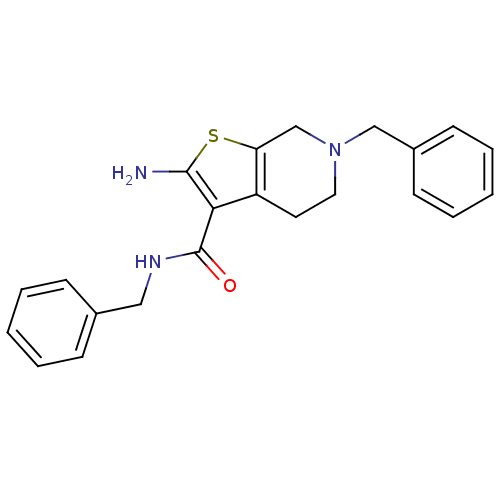 (2-Amino-N,6-dibenzyl-4,5,6,7-tetrahydrothieno[2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304465 (2-Amino-6-(tert-butoxycarbonyl)-4,5,6,7-tetrahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304466 (2-Amino-N-benzyl-6-methyl-4,5,6,7-tetrahydrothieno...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304467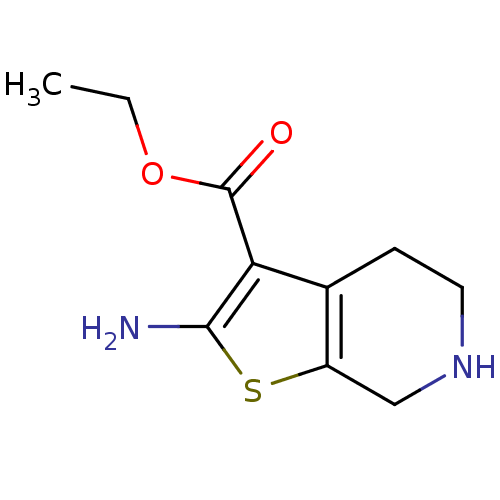 (CHEMBL595965 | Ethyl 2-amino-4,5,6,7-tetrahydrothi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304468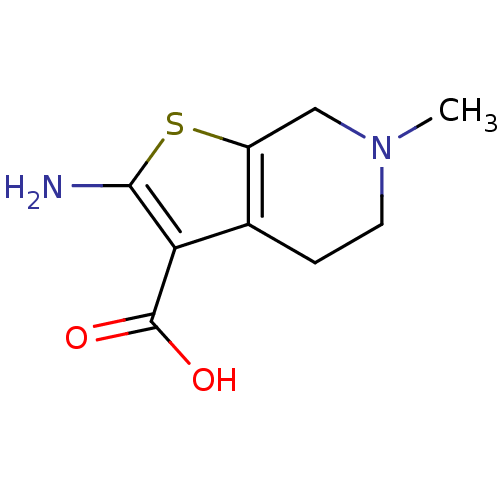 (2-Amino-6-methyl-4,5,6,7-tetrahydrothieno[2,3-c]py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304469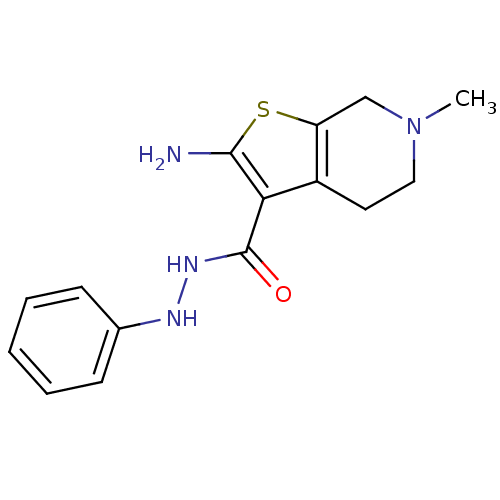 (2-Amino-6-methyl-N'-phenyl-4,5,6,7-tetrahydrothien...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304470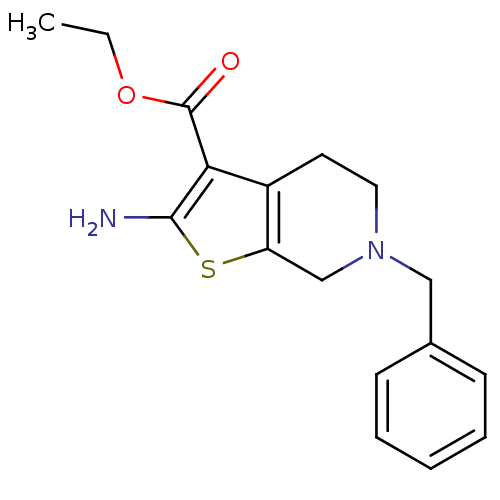 (CHEMBL592943 | Ethyl 2-amino-6-benzyl-4,5,6,7-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304471 ((R)-Ethyl 2-amino-6-(2-(tert-butoxycarbonyl(methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304472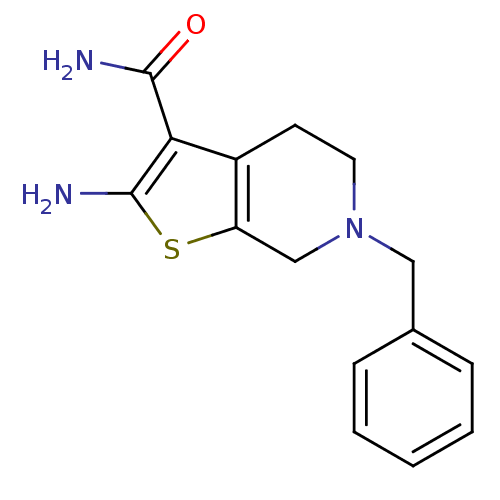 (2-Amino-6-benzyl-4,5,6,7-tetrahydrothieno[2,3-c]py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304473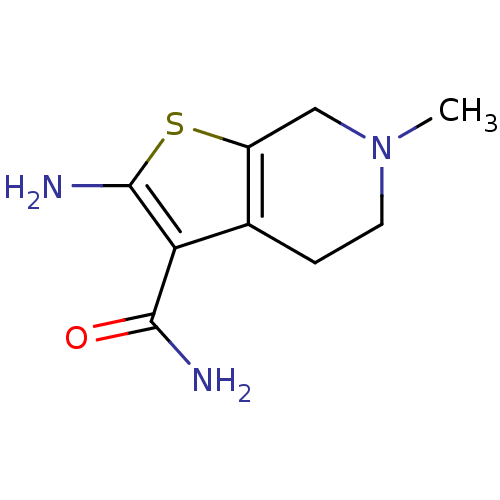 (2-Amino-6-methyl-4,5,6,7-tetrahydrothieno[2,3-c]py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304474 (2-Amino-6-methyl-4,5,6,7-tetrahydrothieno[2,3-c]py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304475 (CHEMBL595295 | tert-Butyl 2-amino-3-cyano-4,5-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304476 (2-Amino-6-benzyl-4,5,6,7-tetrahydrothieno[2,3-c]py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304477 (2-Amino-6-benzyl-4,5,6,7-tetrahydrothieno[2,3-c]py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304478 ((R)-Ethyl 2-amino-6-(2-(tert-butoxycarbonylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304479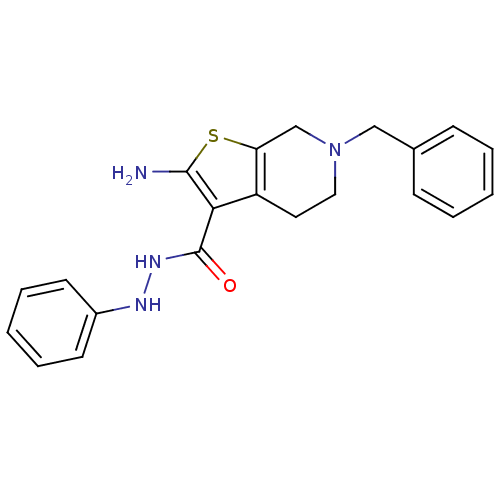 (2-Amino-6-benzyl-N'-phenyl-4,5,6,7-tetrahydrothien...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304480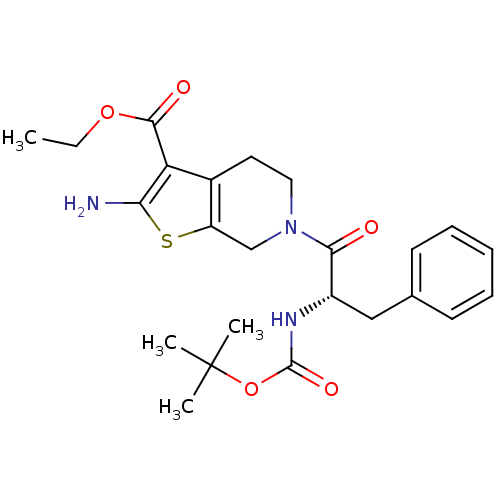 ((S)-Ethyl 2-amino-6-(2-(tert-butoxycarbonylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304481 (2-Amino-6-methyl-4,5,6,7-tetrahydrothieno[2,3-c]py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080550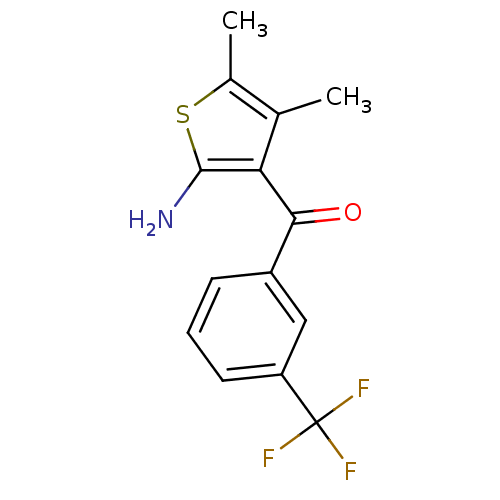 ((2-Amino-4,5-dimethyl-thiophen-3-yl)-(3-trifluorom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304482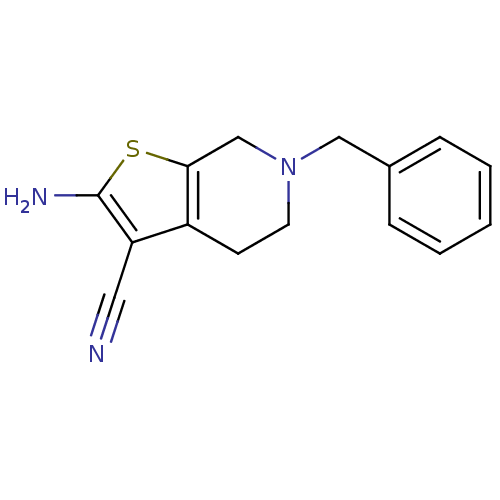 (2-Amino-6-benzyl-4,5,6,7-tetrahydrothieno[2,3-c]py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304483 (2-Amino-6-benzyl-N-(3-(trifluoromethyl)benzyl)-4,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080545 ((2-Amino-6-benzyl-4,5,6,7-tetrahydro-thieno[2,3-c]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304484 (CHEMBL594791 | Ethyl 2-amino-6-methyl-4,5,6,7-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304485 (2-Amino-6-benzyl-N-(3-chlorobenzyl)-4,5,6,7-tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Allosteric modulatory activity at human adenosine A1 receptor expressed in CHOk1 cells assessed as drug level causing half maximal allosteric effect ... | Bioorg Med Chem 17: 7353-61 (2009) Article DOI: 10.1016/j.bmc.2009.08.024 BindingDB Entry DOI: 10.7270/Q2H9959W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50263179 (CHEMBL4096796) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Monash Institute of Pharmaceutical Sciences , Monash University , Parkville , Victoria 3052 , Australia. Curated by ChEMBL | Assay Description Agonist activity at human A1AR expressed in FlpIn-CHO cells assessed as intracellular calcium mobilization by Fluo-4 dye-based fluorescence assay | J Med Chem 61: 2087-2103 (2018) Article DOI: 10.1021/acs.jmedchem.8b00047 BindingDB Entry DOI: 10.7270/Q21J9D7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50263180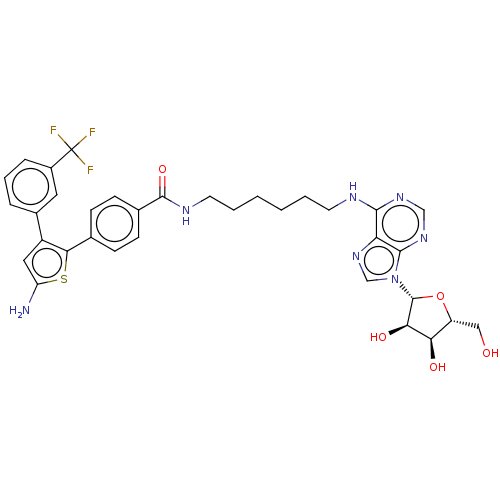 (CHEMBL4081224) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 447 | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Monash Institute of Pharmaceutical Sciences , Monash University , Parkville , Victoria 3052 , Australia. Curated by ChEMBL | Assay Description Agonist activity at human A2BAR expressed in FlpIn-CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation preincubated for 40 min... | J Med Chem 61: 2087-2103 (2018) Article DOI: 10.1021/acs.jmedchem.8b00047 BindingDB Entry DOI: 10.7270/Q21J9D7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 130 total ) | Next | Last >> |