

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167698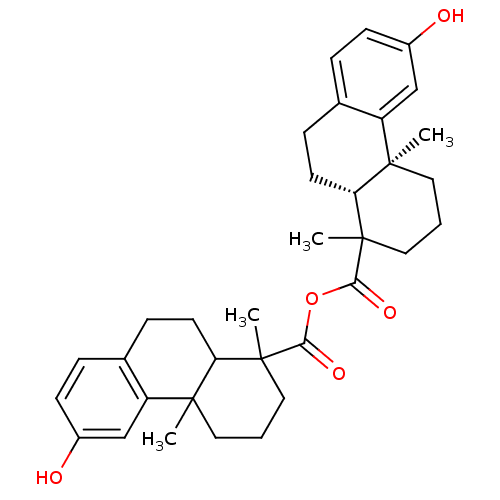 (13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167698 (13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167700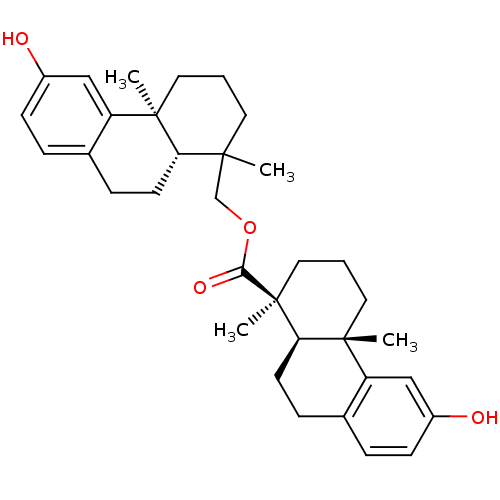 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167700 ((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-alpha in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50126018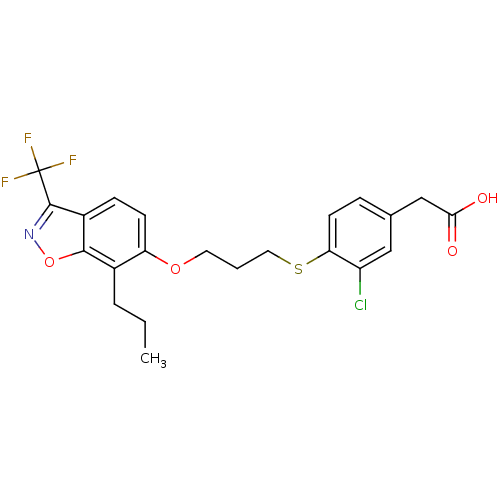 (2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167695 (13-[13-hydroxy-2,6-dimethyl-(2S,6S,7R)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50126018 (2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167699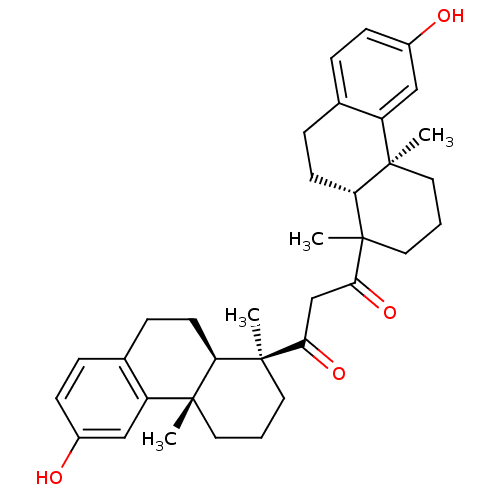 (1-((9R,10S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-alpha in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167695 (13-[13-hydroxy-2,6-dimethyl-(2S,6S,7R)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-alpha in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167699 (1-((9R,10S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167696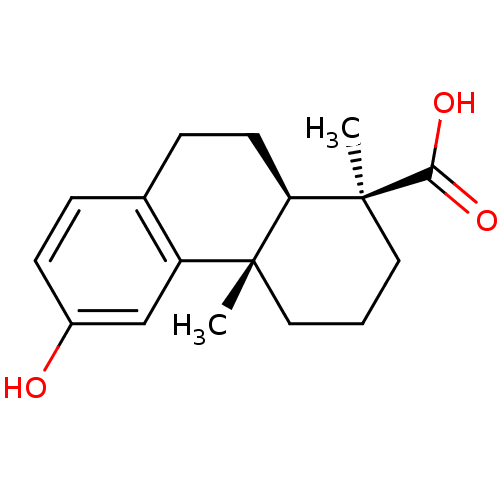 ((1S,4aS,10aR)-1,2,3,4,4a,9,10,10a-octahydro-6-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167696 ((1S,4aS,10aR)-1,2,3,4,4a,9,10,10a-octahydro-6-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-alpha in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167698 (13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-alpha in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20177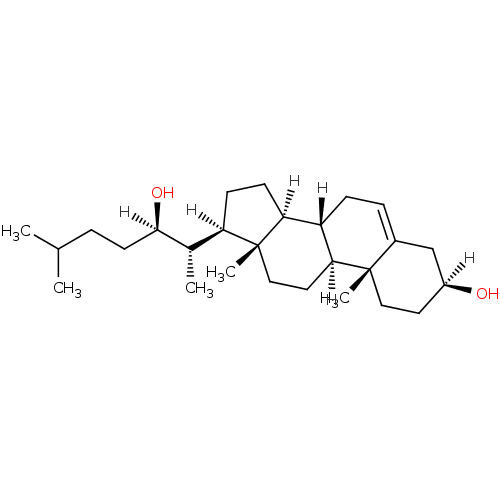 ((1S,2R,5S,10S,11S,14R,15S)-14-[(2S,3R)-3-hydroxy-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-alpha | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50126018 (2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-alpha | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-alpha | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167698 (13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-beta | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-beta | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20177 ((1S,2R,5S,10S,11S,14R,15S)-14-[(2S,3R)-3-hydroxy-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-beta | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167699 (1-((9R,10S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-alpha | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-alpha | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167698 (13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-alpha | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-alpha in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50167696 ((1S,4aS,10aR)-1,2,3,4,4a,9,10,10a-octahydro-6-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-alpha | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167696 ((1S,4aS,10aR)-1,2,3,4,4a,9,10,10a-octahydro-6-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-beta | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50126018 (2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-alpha in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-beta in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-beta | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167698 (13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-beta in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-beta in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50126018 (2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-alpha | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50126018 (2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-beta in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50126018 (2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-beta | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||