 Found 387 hits with Last Name = 'bansal' and Initial = 's'
Found 387 hits with Last Name = 'bansal' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251493

((2S,4S)-1-(2-(1-(4-cyano-3,5-difluorophenyl)piperi...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CNC1CCN(CC1)c1cc(F)c(C#N)c(F)c1 |r| Show InChI InChI=1S/C19H20F3N5O/c20-12-5-15(8-23)27(11-12)19(28)10-25-13-1-3-26(4-2-13)14-6-17(21)16(9-24)18(22)7-14/h6-7,12-13,15,25H,1-5,10-11H2/t12-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492667

(CHEMBL2407709)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(SCC1=O)c1cccc(Cl)c1 Show InChI InChI=1S/C15H13ClN2O3S2/c16-11-3-1-2-10(8-11)15-18(14(19)9-22-15)12-4-6-13(7-5-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492669

(CHEMBL2407710)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(SCC1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H13ClN2O3S2/c16-11-3-1-10(2-4-11)15-18(14(19)9-22-15)12-5-7-13(8-6-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251494
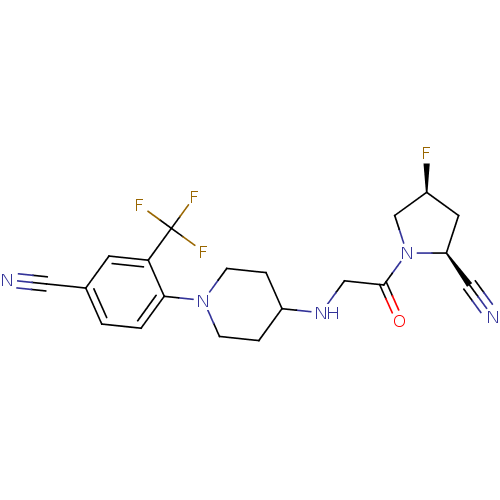
((2S,4S)-1-(2-(1-(4-cyano-2-(trifluoromethyl)phenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CNC1CCN(CC1)c1ccc(cc1C(F)(F)F)C#N |r| Show InChI InChI=1S/C20H21F4N5O/c21-14-8-16(10-26)29(12-14)19(30)11-27-15-3-5-28(6-4-15)18-2-1-13(9-25)7-17(18)20(22,23)24/h1-2,7,14-16,27H,3-6,8,11-12H2/t14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492665

(CHEMBL2407708)Show InChI InChI=1S/C15H13ClN2O3S2/c16-13-4-2-1-3-12(13)15-18(14(19)9-22-15)10-5-7-11(8-6-10)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492664

(CHEMBL2407711)Show InChI InChI=1S/C15H13BrN2O3S2/c16-13-4-2-1-3-12(13)15-18(14(19)9-22-15)10-5-7-11(8-6-10)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251495
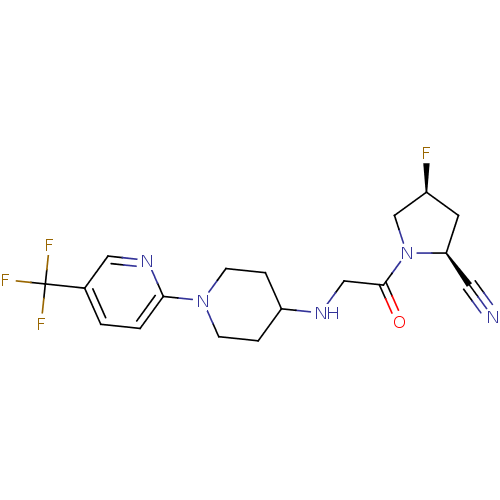
((2S,4S)-4-fluoro-1-(2-(1-(5-(trifluoromethyl)pyrid...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CNC1CCN(CC1)c1ccc(cn1)C(F)(F)F |r| Show InChI InChI=1S/C18H21F4N5O/c19-13-7-15(8-23)27(11-13)17(28)10-24-14-3-5-26(6-4-14)16-2-1-12(9-25-16)18(20,21)22/h1-2,9,13-15,24H,3-7,10-11H2/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492671

(CHEMBL2407713)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(SCC1=O)c1ccc(Br)cc1 Show InChI InChI=1S/C15H13BrN2O3S2/c16-11-3-1-10(2-4-11)15-18(14(19)9-22-15)12-5-7-13(8-6-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492672
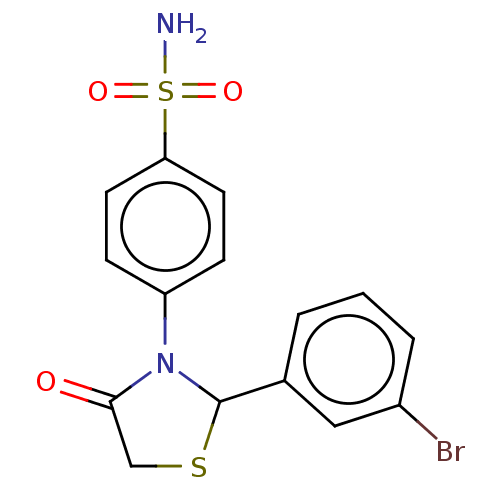
(CHEMBL2407712)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(SCC1=O)c1cccc(Br)c1 Show InChI InChI=1S/C15H13BrN2O3S2/c16-11-3-1-2-10(8-11)15-18(14(19)9-22-15)12-4-6-13(7-5-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492673
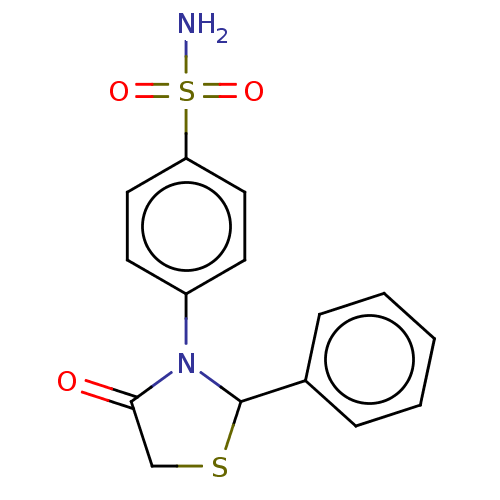
(CHEMBL2216818)Show InChI InChI=1S/C15H14N2O3S2/c16-22(19,20)13-8-6-12(7-9-13)17-14(18)10-21-15(17)11-4-2-1-3-5-11/h1-9,15H,10H2,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492671

(CHEMBL2407713)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(SCC1=O)c1ccc(Br)cc1 Show InChI InChI=1S/C15H13BrN2O3S2/c16-11-3-1-10(2-4-11)15-18(14(19)9-22-15)12-5-7-13(8-6-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251517
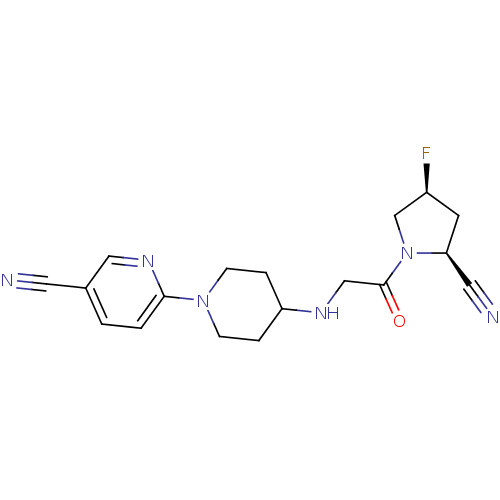
(6-(4-(2-((2S,4S)-2-cyano-4-fluoropyrrolidin-1-yl)-...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CNC1CCN(CC1)c1ccc(cn1)C#N |r| Show InChI InChI=1S/C18H21FN6O/c19-14-7-16(9-21)25(12-14)18(26)11-22-15-3-5-24(6-4-15)17-2-1-13(8-20)10-23-17/h1-2,10,14-16,22H,3-7,11-12H2/t14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492664

(CHEMBL2407711)Show InChI InChI=1S/C15H13BrN2O3S2/c16-13-4-2-1-3-12(13)15-18(14(19)9-22-15)10-5-7-11(8-6-10)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492668
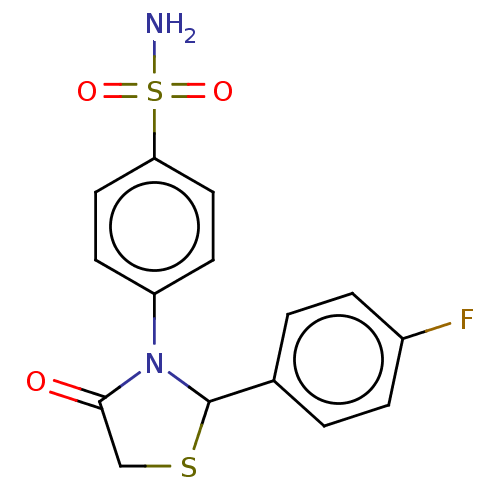
(CHEMBL2407707)Show InChI InChI=1S/C15H13FN2O3S2/c16-11-3-1-10(2-4-11)15-18(14(19)9-22-15)12-5-7-13(8-6-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492670
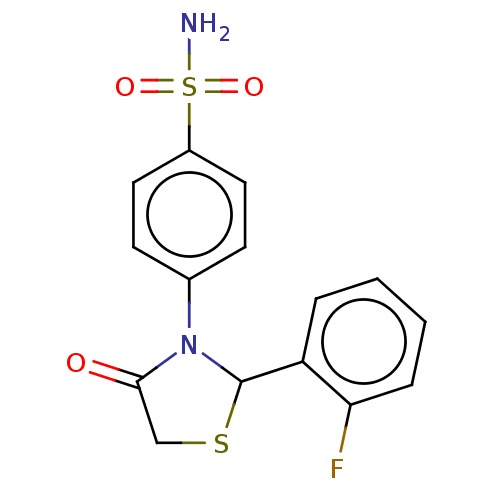
(CHEMBL2407714)Show InChI InChI=1S/C15H13FN2O3S2/c16-13-4-2-1-3-12(13)15-18(14(19)9-22-15)10-5-7-11(8-6-10)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492668

(CHEMBL2407707)Show InChI InChI=1S/C15H13FN2O3S2/c16-11-3-1-10(2-4-11)15-18(14(19)9-22-15)12-5-7-13(8-6-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492669

(CHEMBL2407710)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(SCC1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H13ClN2O3S2/c16-11-3-1-10(2-4-11)15-18(14(19)9-22-15)12-5-7-13(8-6-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492665

(CHEMBL2407708)Show InChI InChI=1S/C15H13ClN2O3S2/c16-13-4-2-1-3-12(13)15-18(14(19)9-22-15)10-5-7-11(8-6-10)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492670

(CHEMBL2407714)Show InChI InChI=1S/C15H13FN2O3S2/c16-13-4-2-1-3-12(13)15-18(14(19)9-22-15)10-5-7-11(8-6-10)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492666
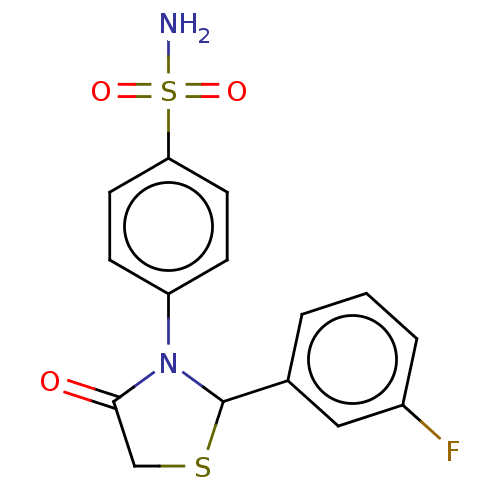
(CHEMBL2407715)Show InChI InChI=1S/C15H13FN2O3S2/c16-11-3-1-2-10(8-11)15-18(14(19)9-22-15)12-4-6-13(7-5-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492666

(CHEMBL2407715)Show InChI InChI=1S/C15H13FN2O3S2/c16-11-3-1-2-10(8-11)15-18(14(19)9-22-15)12-4-6-13(7-5-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of carbonic anhydrase 9 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492673

(CHEMBL2216818)Show InChI InChI=1S/C15H14N2O3S2/c16-22(19,20)13-8-6-12(7-9-13)17-14(18)10-21-15(17)11-4-2-1-3-5-11/h1-9,15H,10H2,(H2,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251518
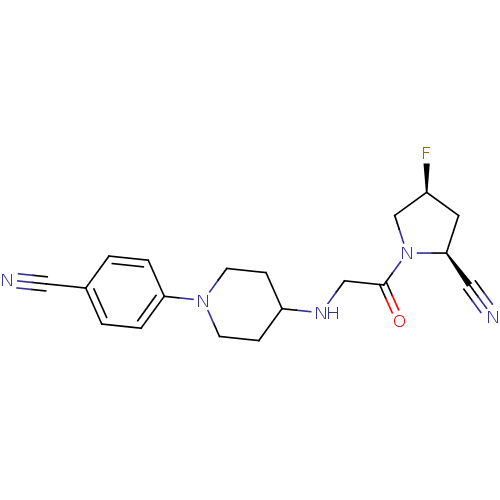
((2S,4S)-1-(2-(1-(4-cyanophenyl)piperidin-4-ylamino...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CNC1CCN(CC1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C19H22FN5O/c20-15-9-18(11-22)25(13-15)19(26)12-23-16-5-7-24(8-6-16)17-3-1-14(10-21)2-4-17/h1-4,15-16,18,23H,5-9,12-13H2/t15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50492673

(CHEMBL2216818)Show InChI InChI=1S/C15H14N2O3S2/c16-22(19,20)13-8-6-12(7-9-13)17-14(18)10-21-15(17)11-4-2-1-3-5-11/h1-9,15H,10H2,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 1 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492672

(CHEMBL2407712)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(SCC1=O)c1cccc(Br)c1 Show InChI InChI=1S/C15H13BrN2O3S2/c16-11-3-1-2-10(8-11)15-18(14(19)9-22-15)12-4-6-13(7-5-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492667

(CHEMBL2407709)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(SCC1=O)c1cccc(Cl)c1 Show InChI InChI=1S/C15H13ClN2O3S2/c16-11-3-1-2-10(8-11)15-18(14(19)9-22-15)12-4-6-13(7-5-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 1 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50492665

(CHEMBL2407708)Show InChI InChI=1S/C15H13ClN2O3S2/c16-13-4-2-1-3-12(13)15-18(14(19)9-22-15)10-5-7-11(8-6-10)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 737 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 1 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50492669

(CHEMBL2407710)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(SCC1=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C15H13ClN2O3S2/c16-11-3-1-10(2-4-11)15-18(14(19)9-22-15)12-5-7-13(8-6-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 742 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 1 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50492667

(CHEMBL2407709)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(SCC1=O)c1cccc(Cl)c1 Show InChI InChI=1S/C15H13ClN2O3S2/c16-11-3-1-2-10(8-11)15-18(14(19)9-22-15)12-4-6-13(7-5-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 838 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 1 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50492664

(CHEMBL2407711)Show InChI InChI=1S/C15H13BrN2O3S2/c16-13-4-2-1-3-12(13)15-18(14(19)9-22-15)10-5-7-11(8-6-10)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 936 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 1 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50492670

(CHEMBL2407714)Show InChI InChI=1S/C15H13FN2O3S2/c16-13-4-2-1-3-12(13)15-18(14(19)9-22-15)10-5-7-11(8-6-10)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 1 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50492671

(CHEMBL2407713)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(SCC1=O)c1ccc(Br)cc1 Show InChI InChI=1S/C15H13BrN2O3S2/c16-11-3-1-10(2-4-11)15-18(14(19)9-22-15)12-5-7-13(8-6-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 1 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50492668

(CHEMBL2407707)Show InChI InChI=1S/C15H13FN2O3S2/c16-11-3-1-10(2-4-11)15-18(14(19)9-22-15)12-5-7-13(8-6-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 1 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50492672

(CHEMBL2407712)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(SCC1=O)c1cccc(Br)c1 Show InChI InChI=1S/C15H13BrN2O3S2/c16-11-3-1-2-10(8-11)15-18(14(19)9-22-15)12-4-6-13(7-5-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 1 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50492666

(CHEMBL2407715)Show InChI InChI=1S/C15H13FN2O3S2/c16-11-3-1-2-10(8-11)15-18(14(19)9-22-15)12-4-6-13(7-5-12)23(17,20)21/h1-8,15H,9H2,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 1 (unknown origin) preincubated for 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 66: 372-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.003
BindingDB Entry DOI: 10.7270/Q2K93BF3 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50277112

(3-[1-Oxo-6-(1,2,3,4-tetrahydro-benzo[4,5]imidazo[1...)Show SMILES OC(=O)CC(N1CCc2cc(Oc3cccc4n5CCCNc5nc34)ccc2C1=O)c1cccnc1 Show InChI InChI=1S/C27H25N5O4/c33-24(34)15-22(18-4-2-10-28-16-18)31-13-9-17-14-19(7-8-20(17)26(31)35)36-23-6-1-5-21-25(23)30-27-29-11-3-12-32(21)27/h1-2,4-8,10,14,16,22H,3,9,11-13,15H2,(H,29,30)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human integrin alphavbeta3 receptor by TRF assay |
Bioorg Med Chem Lett 19: 352-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.074
BindingDB Entry DOI: 10.7270/Q2N016C9 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50208999
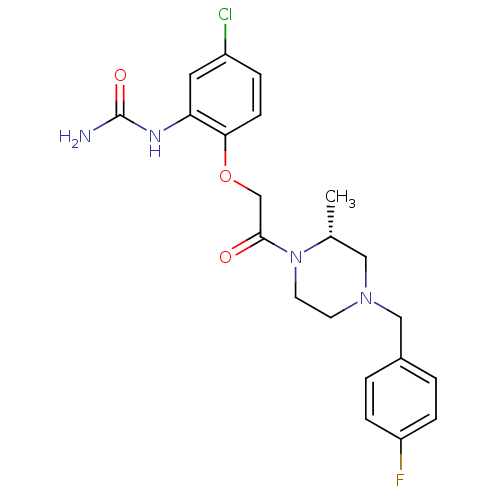
((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O |r| Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR1 in THP1 cells assessed as inhibition of MIP-1alpha-induced chemotaxis after 3 hrs |
J Med Chem 52: 1295-301 (2010)
Article DOI: 10.1021/jm801416q
BindingDB Entry DOI: 10.7270/Q25X28Z3 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50277111

(3-Benzo[1,3]dioxol-5-yl-3-[1-oxo-6-(1,2,3,4-tetrah...)Show SMILES OC(=O)CC(N1CCc2cc(Oc3cccc4n5CCCNc5nc34)ccc2C1=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H26N4O6/c34-26(35)15-22(18-5-8-23-25(14-18)38-16-37-23)32-12-9-17-13-19(6-7-20(17)28(32)36)39-24-4-1-3-21-27(24)31-29-30-10-2-11-33(21)29/h1,3-8,13-14,22H,2,9-12,15-16H2,(H,30,31)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human integrin alphavbeta3 receptor by TRF assay |
Bioorg Med Chem Lett 19: 352-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.074
BindingDB Entry DOI: 10.7270/Q2N016C9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Manipal College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of MAO-A in bovine brain mitochondria using serotonin as substrate preincubated for 30 mins measured after 30 mins by spectrofluorimetric ... |
Bioorg Med Chem Lett 25: 5281-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.048
BindingDB Entry DOI: 10.7270/Q2M90BH5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50277112

(3-[1-Oxo-6-(1,2,3,4-tetrahydro-benzo[4,5]imidazo[1...)Show SMILES OC(=O)CC(N1CCc2cc(Oc3cccc4n5CCCNc5nc34)ccc2C1=O)c1cccnc1 Show InChI InChI=1S/C27H25N5O4/c33-24(34)15-22(18-4-2-10-28-16-18)31-13-9-17-14-19(7-8-20(17)26(31)35)36-23-6-1-5-21-25(23)30-27-29-11-3-12-32(21)27/h1-2,4-8,10,14,16,22H,3,9,11-13,15H2,(H,29,30)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human integrin alphavbeta5 receptor by TRF assay |
Bioorg Med Chem Lett 19: 352-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.074
BindingDB Entry DOI: 10.7270/Q2N016C9 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50208999

((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O |r| Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR1 expressed in HEK293 cells |
J Med Chem 52: 1295-301 (2010)
Article DOI: 10.1021/jm801416q
BindingDB Entry DOI: 10.7270/Q25X28Z3 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50293055
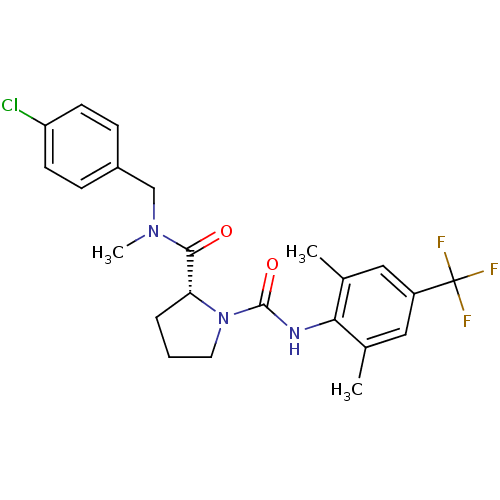
((R)-N2-(4-Chlorobenzyl)-N1-[2,6-dimethyl-4-(triflu...)Show SMILES CN(Cc1ccc(Cl)cc1)C(=O)[C@H]1CCCN1C(=O)Nc1c(C)cc(cc1C)C(F)(F)F |r| Show InChI InChI=1S/C23H25ClF3N3O2/c1-14-11-17(23(25,26)27)12-15(2)20(14)28-22(32)30-10-4-5-19(30)21(31)29(3)13-16-6-8-18(24)9-7-16/h6-9,11-12,19H,4-5,10,13H2,1-3H3,(H,28,32)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR1 in THP1 cells assessed as inhibition of MIP-1alpha-induced chemotaxis after 3 hrs |
J Med Chem 52: 1295-301 (2010)
Article DOI: 10.1021/jm801416q
BindingDB Entry DOI: 10.7270/Q25X28Z3 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50277098
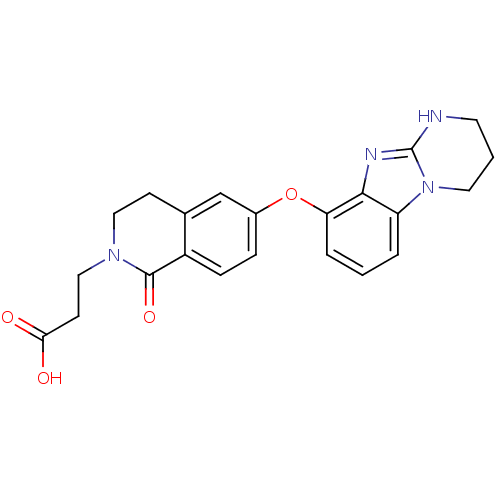
(3-[1-Oxo-6-(1,2,3,4-tetrahydro-benzo[4,5]imidazo[1...)Show SMILES OC(=O)CCN1CCc2cc(Oc3cccc4n5CCCNc5nc34)ccc2C1=O Show InChI InChI=1S/C22H22N4O4/c27-19(28)8-12-25-11-7-14-13-15(5-6-16(14)21(25)29)30-18-4-1-3-17-20(18)24-22-23-9-2-10-26(17)22/h1,3-6,13H,2,7-12H2,(H,23,24)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human integrin alphavbeta3 receptor by TRF assay |
Bioorg Med Chem Lett 19: 352-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.074
BindingDB Entry DOI: 10.7270/Q2N016C9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50293055

((R)-N2-(4-Chlorobenzyl)-N1-[2,6-dimethyl-4-(triflu...)Show SMILES CN(Cc1ccc(Cl)cc1)C(=O)[C@H]1CCCN1C(=O)Nc1c(C)cc(cc1C)C(F)(F)F |r| Show InChI InChI=1S/C23H25ClF3N3O2/c1-14-11-17(23(25,26)27)12-15(2)20(14)28-22(32)30-10-4-5-19(30)21(31)29(3)13-16-6-8-18(24)9-7-16/h6-9,11-12,19H,4-5,10,13H2,1-3H3,(H,28,32)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from human recombinant CCR1 expressed in HEK293 cells |
J Med Chem 52: 1295-301 (2010)
Article DOI: 10.1021/jm801416q
BindingDB Entry DOI: 10.7270/Q25X28Z3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data