

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50173287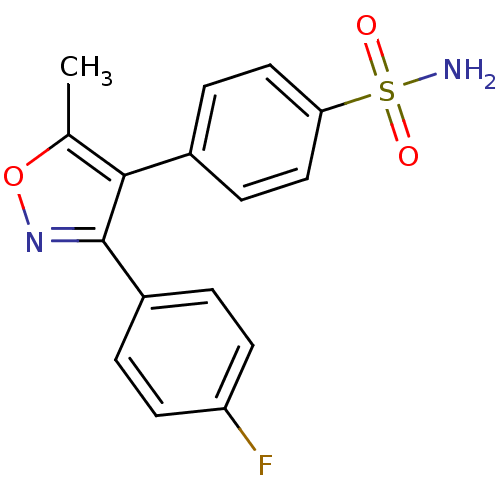 (4-[3-(4-Fluoro-phenyl)-5-methyl-isoxazol-4-yl]-ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
David Geffen School of Medicine at University of California Curated by ChEMBL | Assay Description Tested for prostaglandin E2 production as a function of COX-2 inhibition using endotoxin-treated murine RAW 264.7 macrophages | Bioorg Med Chem Lett 15: 4699-702 (2005) Article DOI: 10.1016/j.bmcl.2005.07.065 BindingDB Entry DOI: 10.7270/Q2H131KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50173288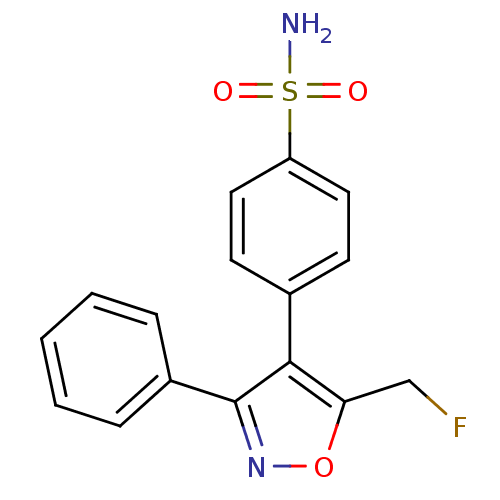 (4-(5-Fluoromethyl-3-phenyl-isoxazol-4-yl)-benzenes...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
David Geffen School of Medicine at University of California Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant cyclooxygenase 2 | Bioorg Med Chem Lett 15: 4699-702 (2005) Article DOI: 10.1016/j.bmcl.2005.07.065 BindingDB Entry DOI: 10.7270/Q2H131KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50173288 (4-(5-Fluoromethyl-3-phenyl-isoxazol-4-yl)-benzenes...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
David Geffen School of Medicine at University of California Curated by ChEMBL | Assay Description Tested for prostaglandin E2 production as a function of COX-2 inhibition using endotoxin-treated murine RAW 264.7 macrophages | Bioorg Med Chem Lett 15: 4699-702 (2005) Article DOI: 10.1016/j.bmcl.2005.07.065 BindingDB Entry DOI: 10.7270/Q2H131KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50173290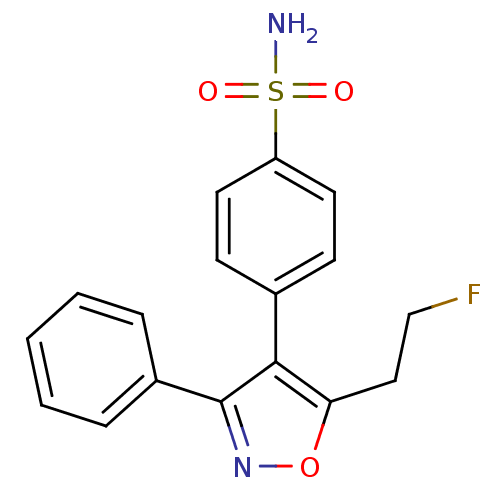 (4-[5-(2-Fluoro-ethyl)-3-phenyl-isoxazol-4-yl]-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
David Geffen School of Medicine at University of California Curated by ChEMBL | Assay Description Tested for prostaglandin E2 production as a function of COX-2 inhibition using endotoxin-treated murine RAW 264.7 macrophages | Bioorg Med Chem Lett 15: 4699-702 (2005) Article DOI: 10.1016/j.bmcl.2005.07.065 BindingDB Entry DOI: 10.7270/Q2H131KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50173289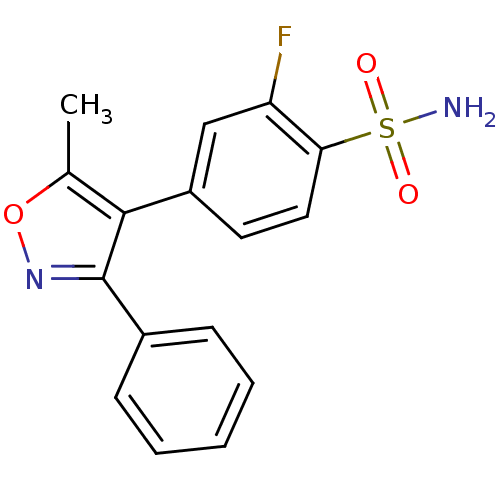 (2-Fluoro-4-(5-methyl-3-phenyl-isoxazol-4-yl)-benze...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
David Geffen School of Medicine at University of California Curated by ChEMBL | Assay Description Tested for prostaglandin E2 production as a function of COX-2 inhibition using endotoxin-treated murine RAW 264.7 macrophages | Bioorg Med Chem Lett 15: 4699-702 (2005) Article DOI: 10.1016/j.bmcl.2005.07.065 BindingDB Entry DOI: 10.7270/Q2H131KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM13063 (4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
David Geffen School of Medicine at University of California Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant cyclooxygenase 2 | Bioorg Med Chem Lett 15: 4699-702 (2005) Article DOI: 10.1016/j.bmcl.2005.07.065 BindingDB Entry DOI: 10.7270/Q2H131KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50173290 (4-[5-(2-Fluoro-ethyl)-3-phenyl-isoxazol-4-yl]-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
David Geffen School of Medicine at University of California Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant cyclooxygenase 2 | Bioorg Med Chem Lett 15: 4699-702 (2005) Article DOI: 10.1016/j.bmcl.2005.07.065 BindingDB Entry DOI: 10.7270/Q2H131KS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||