

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255582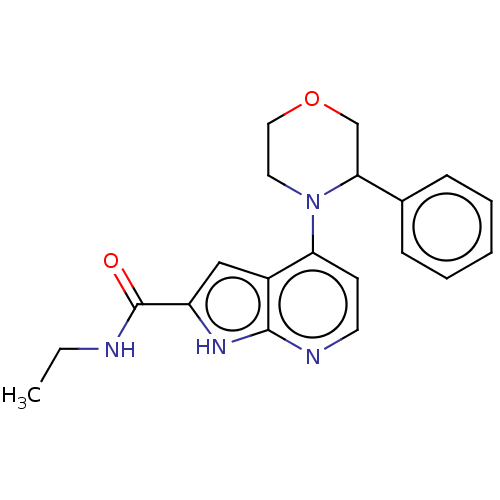 (CHEMBL4078783) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255621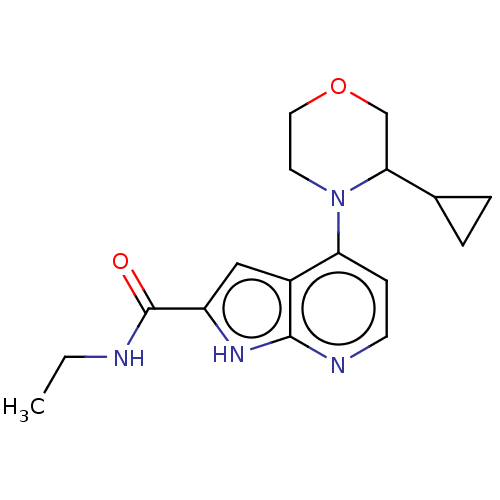 (CHEMBL4070624) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255568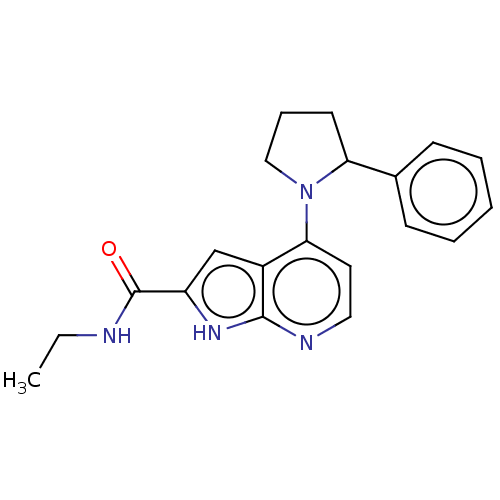 (CHEMBL4091768) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Inhibition of human full length MTH1 using 8-Oxo-2-dGTP as substrate preincubated for 15 mins followed substrate addition measured after 1 hr by PPil... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255566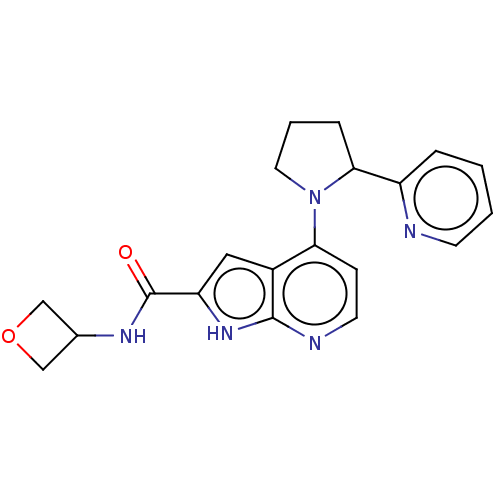 (CHEMBL4094252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255598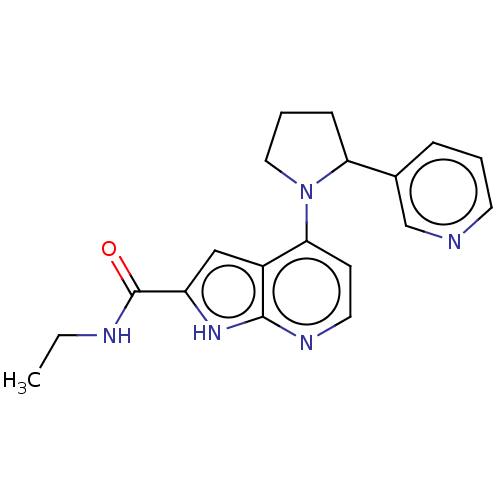 (CHEMBL4064004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255603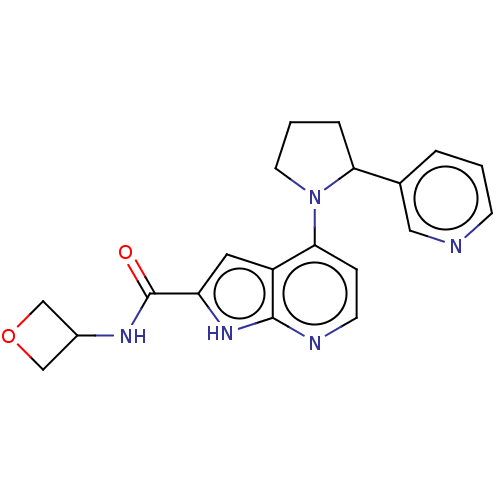 (CHEMBL4083992) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255622 (CHEMBL4067896) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255624 (CHEMBL4101983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255581 (CHEMBL4073623) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Inhibition of human full length MTH1 using 8-Oxo-2-dGTP as substrate preincubated for 15 mins followed substrate addition measured after 1 hr by PPil... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255583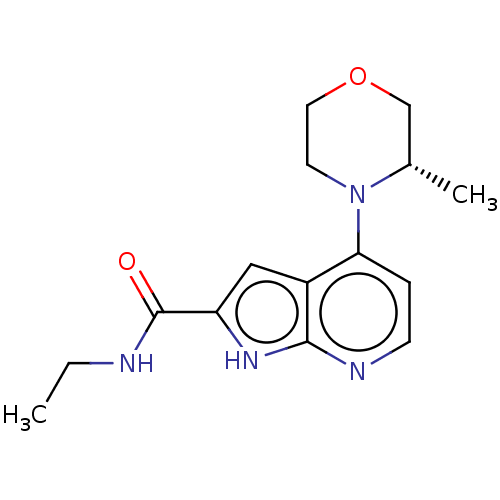 (CHEMBL4096813) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063139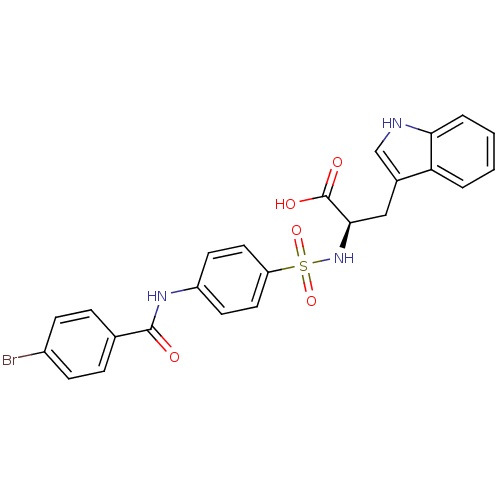 ((R)-2-[4-(4-Bromo-benzoylamino)-benzenesulfonylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50611048 (BAY-091) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PDB UniChem | PDB | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50491906 (CHEMBL2385442) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50491913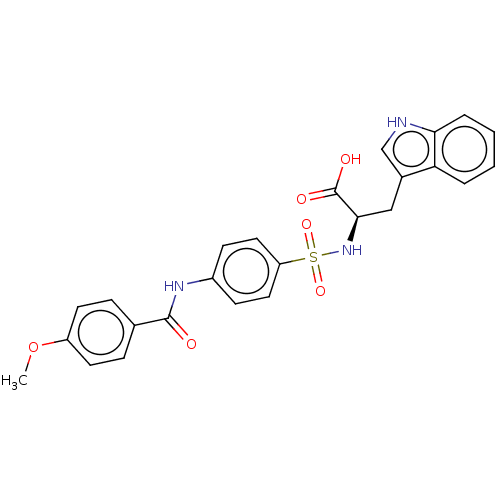 (CHEMBL2385443) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50143581 (7-[4-(4-Fluoro-benzyl)-[1,4]diazepan-1-yl]-2-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AG Curated by ChEMBL | Assay Description Inhibitory activity against human adenosine kinase expressed in E. coli | Bioorg Med Chem Lett 14: 1997-2000 (2004) Article DOI: 10.1016/j.bmcl.2004.01.082 BindingDB Entry DOI: 10.7270/Q2GQ6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50143575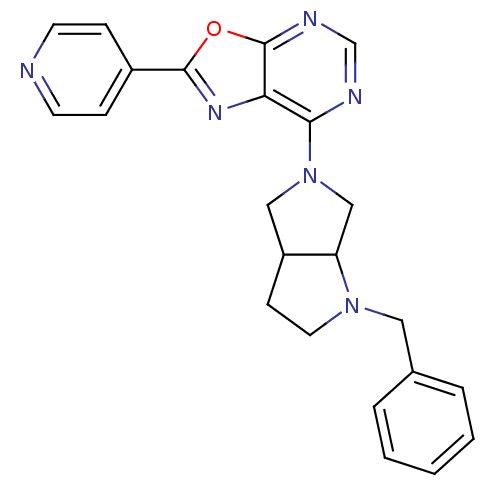 (7-(1-Benzyl-hexahydro-pyrrolo[3,4-b]pyrrol-5-yl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AG Curated by ChEMBL | Assay Description Inhibitory activity against human adenosine kinase expressed in E. coli | Bioorg Med Chem Lett 14: 1997-2000 (2004) Article DOI: 10.1016/j.bmcl.2004.01.082 BindingDB Entry DOI: 10.7270/Q2GQ6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50063139 ((R)-2-[4-(4-Bromo-benzoylamino)-benzenesulfonylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP9 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50491912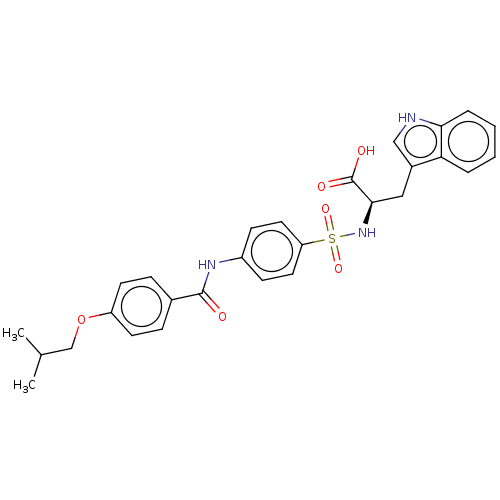 (CHEMBL2385445) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 1 (Homo sapiens (Human)) | BDBM50155671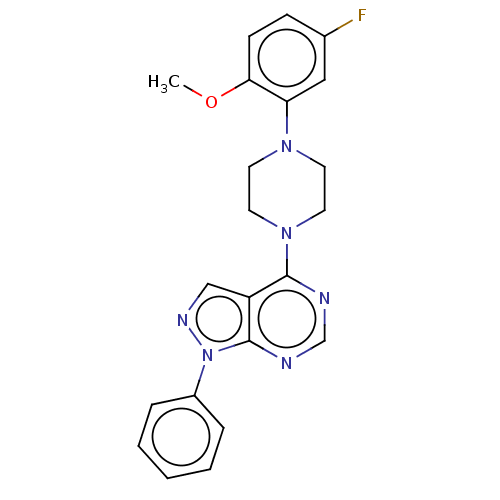 (CHEMBL3780239) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma AG Curated by ChEMBL | Assay Description Inhibition of human GLUT1 expressed in DLD1 cells assessed as ATP production co-incubated with 100 mM glucose for 16 hrs by CellTiter-Glo assay in pr... | Bioorg Med Chem Lett 26: 1732-7 (2016) Article DOI: 10.1016/j.bmcl.2016.02.050 BindingDB Entry DOI: 10.7270/Q2639RM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50491917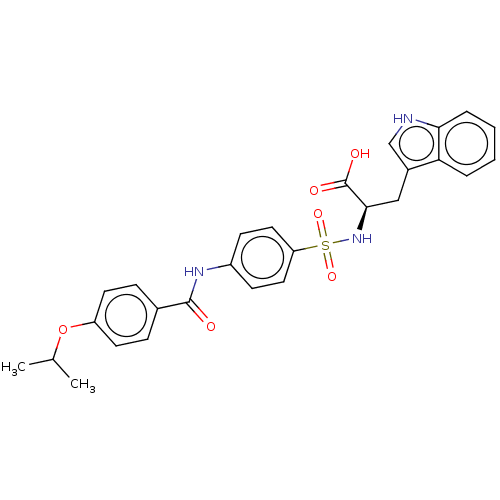 (CHEMBL2385444) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255623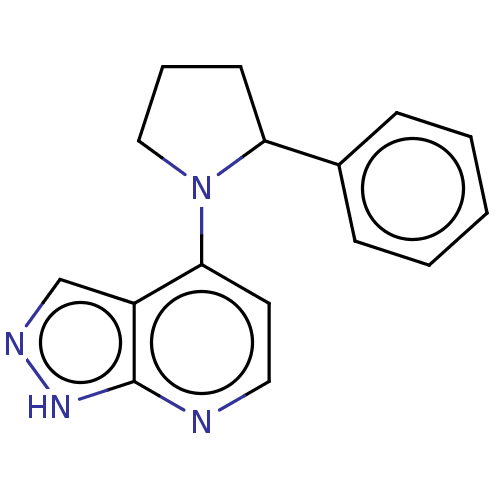 (CHEMBL4072758) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50143574 (7-((4aS,7aS)-6-Benzyl-octahydro-pyrrolo[3,4-b]pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AG Curated by ChEMBL | Assay Description Inhibitory activity against human adenosine kinase expressed in E. coli | Bioorg Med Chem Lett 14: 1997-2000 (2004) Article DOI: 10.1016/j.bmcl.2004.01.082 BindingDB Entry DOI: 10.7270/Q2GQ6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50491906 (CHEMBL2385442) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP9 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50143592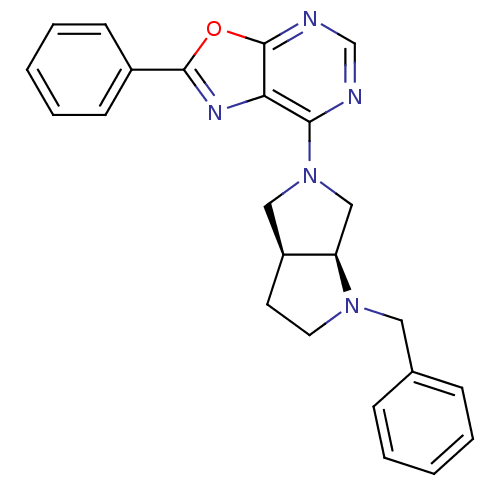 (7-((3aS,6aS)-1-Benzyl-hexahydro-pyrrolo[3,4-b]pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AG Curated by ChEMBL | Assay Description Inhibitory activity against human adenosine kinase expressed in E. coli | Bioorg Med Chem Lett 14: 1997-2000 (2004) Article DOI: 10.1016/j.bmcl.2004.01.082 BindingDB Entry DOI: 10.7270/Q2GQ6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50491907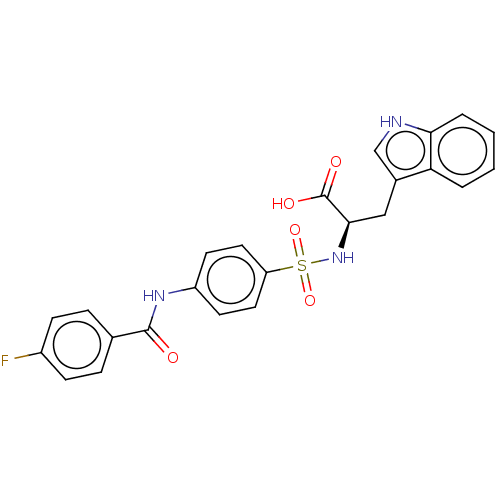 (CHEMBL2385441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255585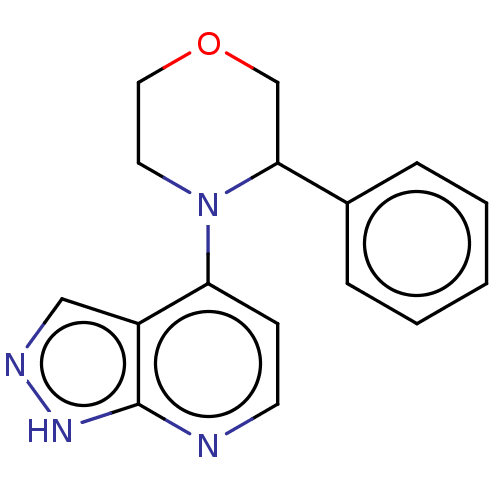 (CHEMBL4094381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50611049 (CHEMBL5280127) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50611048 (BAY-091) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PDB UniChem | PDB | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389389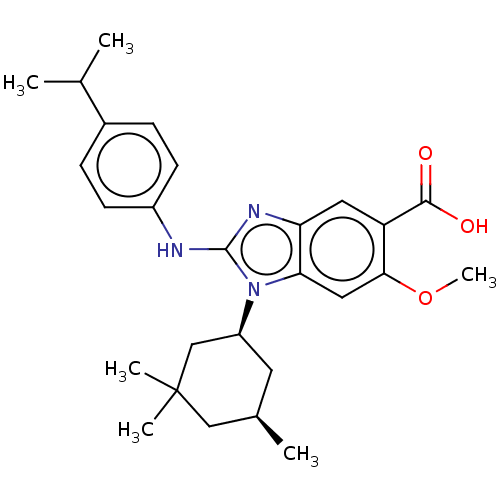 (US9951027, 2-155-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50611049 (CHEMBL5280127) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50491915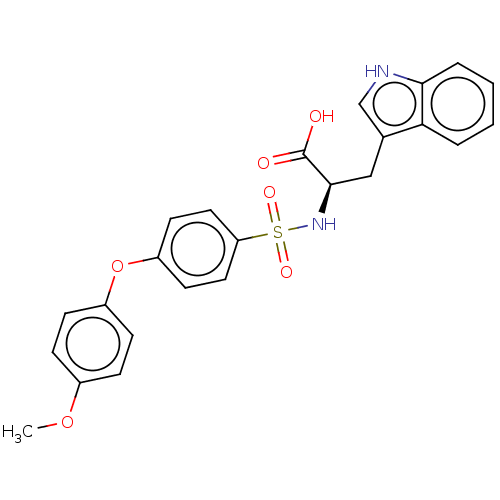 (CHEMBL2385451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389381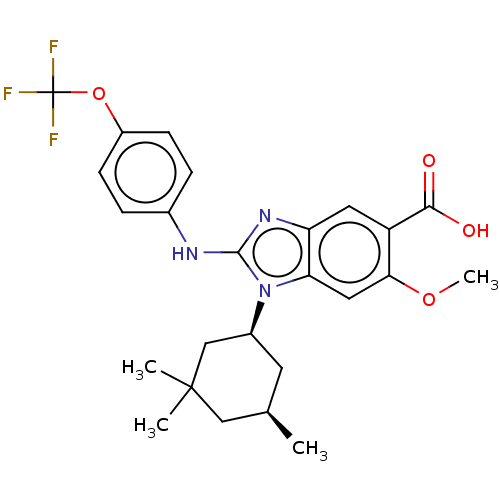 ((+-) 6-methoxy-2-{[4-(trifluoromethoxy)phenyl]amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389324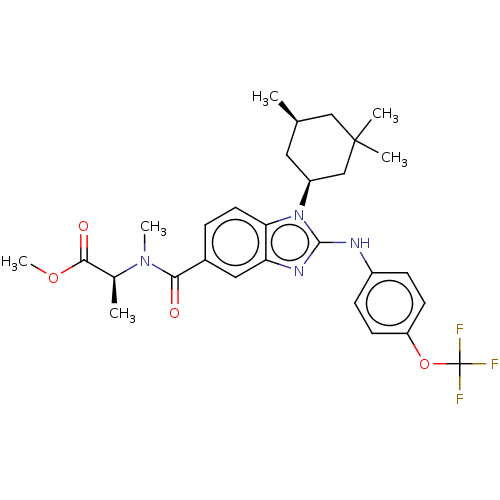 (US9951027, 2-95-2 | US9951027, 2-96-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389364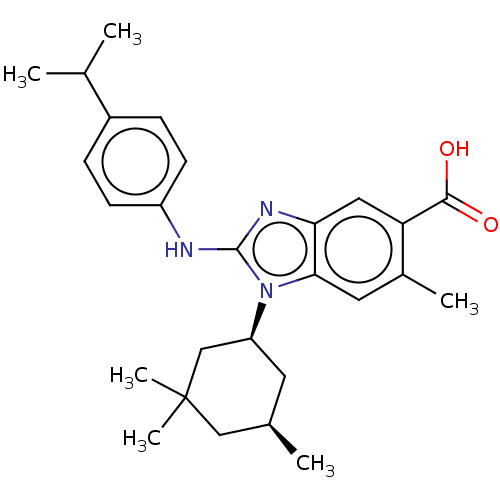 (US9951027, 2-144-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 1 (Homo sapiens (Human)) | BDBM50152749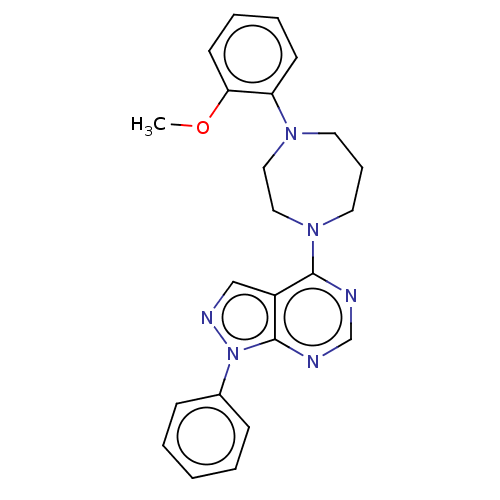 (CHEMBL3781913) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma AG Curated by ChEMBL | Assay Description Inhibition of human GLUT1 expressed in DLD1 cells assessed as ATP production co-incubated with 100 mM glucose for 16 hrs by CellTiter-Glo assay in pr... | Bioorg Med Chem Lett 26: 1732-7 (2016) Article DOI: 10.1016/j.bmcl.2016.02.050 BindingDB Entry DOI: 10.7270/Q2639RM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389290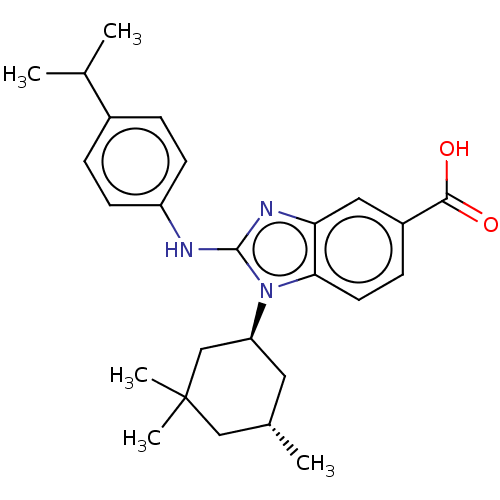 (US9951027, 2-76-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063166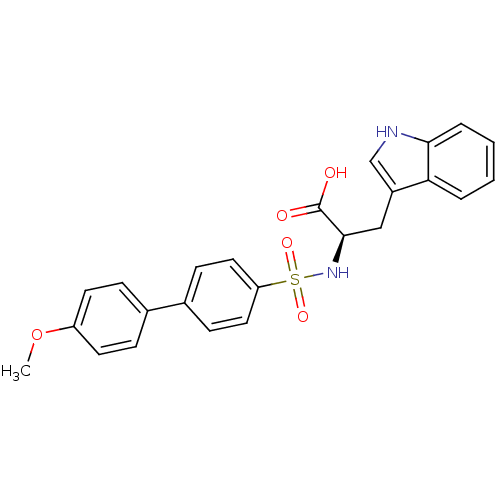 ((R)-3-(1H-Indol-3-yl)-2-(4'-methoxy-biphenyl-4-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50491914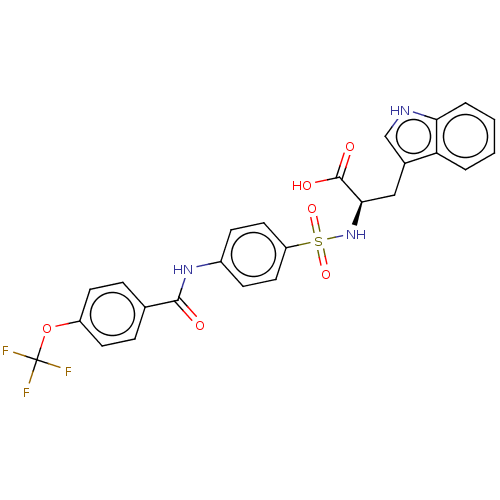 (CHEMBL2385084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390499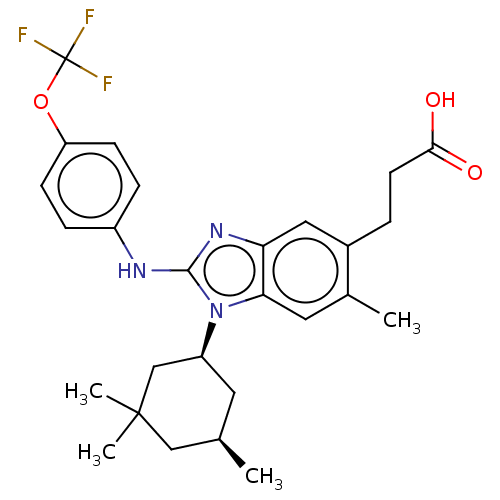 (US10442772, Example 2-162-2 | US9957235, 2-162-1 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... | US Patent US10442772 (2019) BindingDB Entry DOI: 10.7270/Q21R6SVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50610997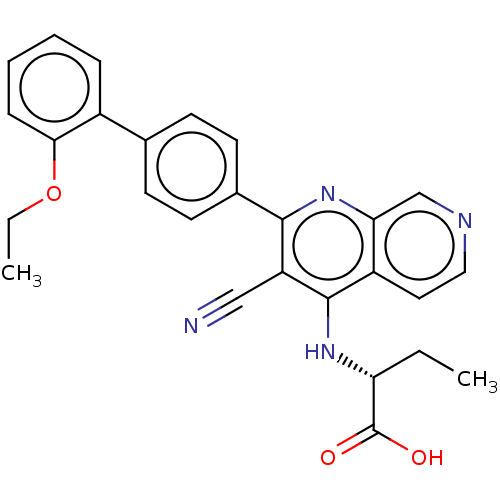 (CHEMBL5276719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 1 (Homo sapiens (Human)) | BDBM50155656 (CHEMBL3781331) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma AG Curated by ChEMBL | Assay Description Inhibition of human GLUT1 expressed in DLD1 cells assessed as ATP production co-incubated with 100 mM glucose for 16 hrs by CellTiter-Glo assay in pr... | Bioorg Med Chem Lett 26: 1732-7 (2016) Article DOI: 10.1016/j.bmcl.2016.02.050 BindingDB Entry DOI: 10.7270/Q2639RM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390498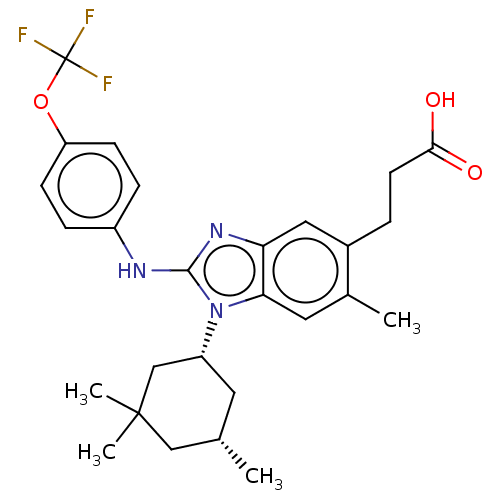 ((�) 3-(6-methyl-2-{[4-(trifluoromethoxy)phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | Bioorg Med Chem 14: 1378-90 (2006) BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255584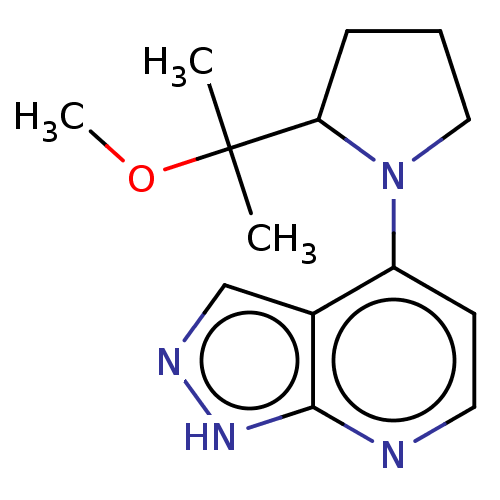 (CHEMBL4089106) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255615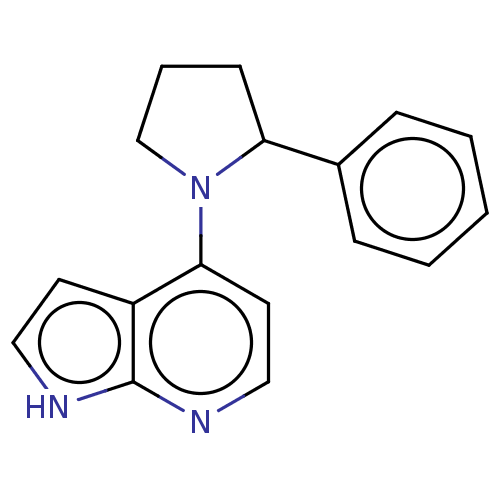 (CHEMBL4061204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50491910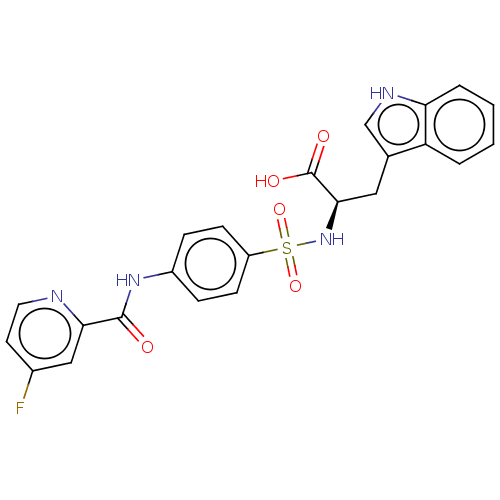 (CHEMBL2385449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 5-phosphate 4-kinase type-2 alpha (Homo sapiens) | BDBM50610997 (CHEMBL5276719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50491909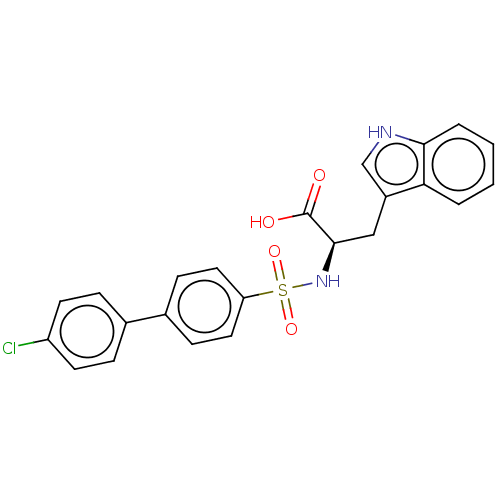 (CHEMBL2385450) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay | J Med Chem 56: 4912-20 (2013) Article DOI: 10.1021/jm400156p BindingDB Entry DOI: 10.7270/Q2959MHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389172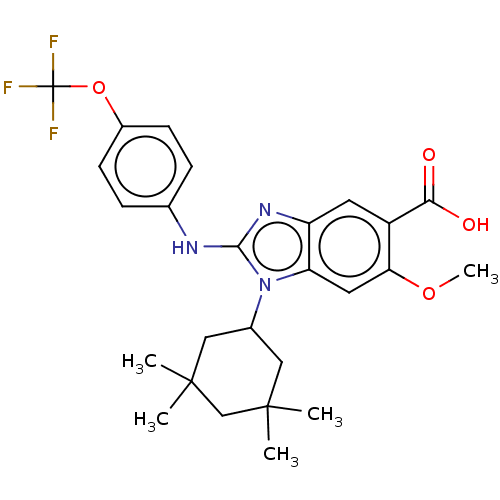 (6-methoxy-1-(3,3,5,5-tetramethylcyclohexyl)-2-{[4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389336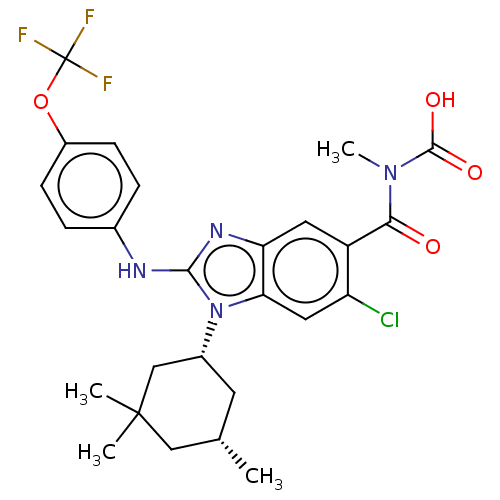 (US9951027, 2-102-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389223 (US9951027, 2-42-2 | US9951027, 2-75-1 | US9951027,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1655 total ) | Next | Last >> |