

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50018014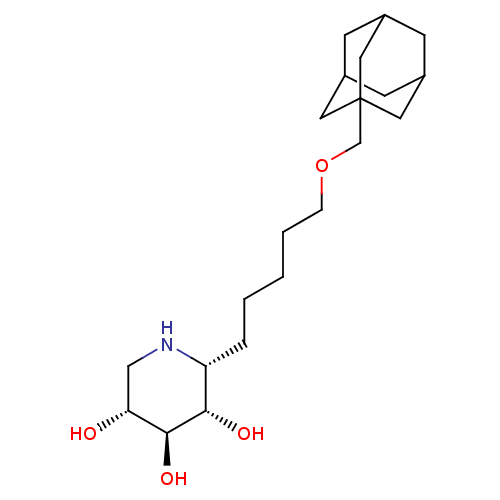 (CHEMBL3289676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50315250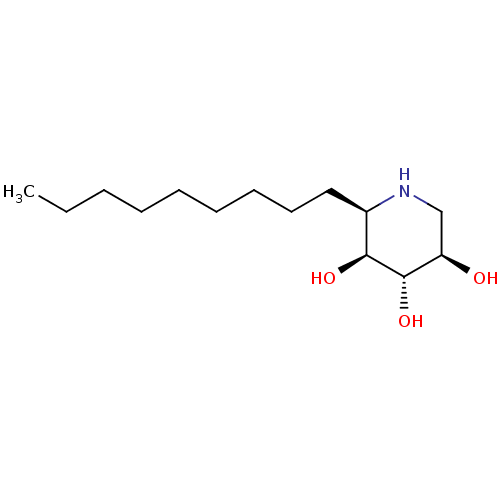 ((2R,3S,4S,5R)-2-nonylpiperidine-3,4,5-triol | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50018018 (CHEMBL3289679) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase using 2,4-dinitrophenyl-beta-D-glucopyranoside as substrate by UV spectrophotometric analysis | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182798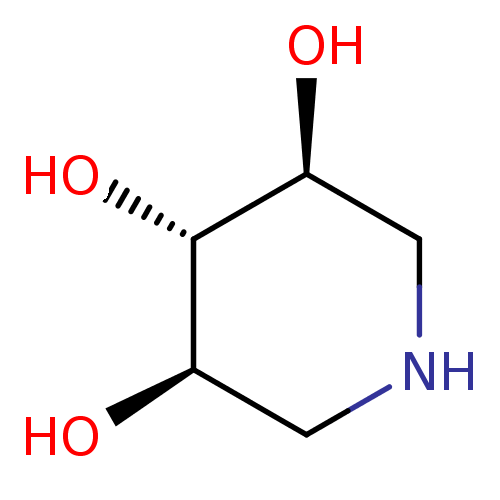 ((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta glucosidase | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50018016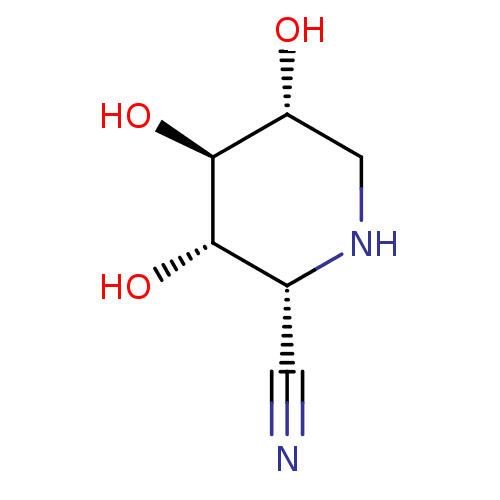 (CHEMBL3289677) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase using 2,4-dinitrophenyl-beta-D-glucopyranoside as substrate by UV spectrophotometric analysis | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM50018018 (CHEMBL3289679) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta glucosidase using 2,4-dinitrophenyl-beta-D-glucopyranoside as substrate measured for 3 mins | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM50182798 ((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta glucosidase | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM50018017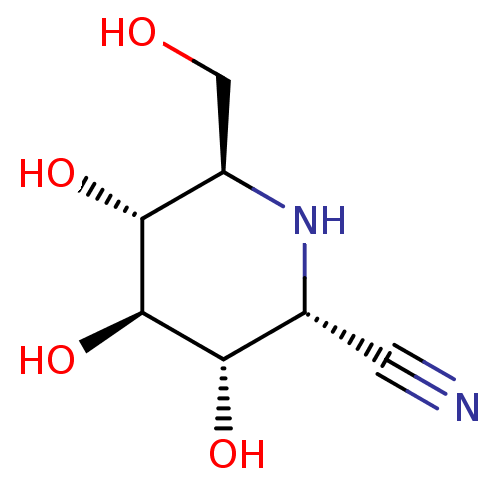 (CHEMBL3289678) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta glucosidase using 2,4-dinitrophenyl-beta-D-glucopyranoside as substrate measured for 3 mins | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Agrobacterium tumefaciens) | BDBM50018016 (CHEMBL3289677) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of Agrobacterium sp. beta glucosidase using 2,4-dinitrophenyl-beta-D-glucopyranoside as substrate measured for 3 mins | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50018017 (CHEMBL3289678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University Graz Curated by ChEMBL | Assay Description Inhibition of human lysosomal beta-glucocerebrosidase using 2,4-dinitrophenyl-beta-D-glucopyranoside as substrate by UV spectrophotometric analysis | Bioorg Med Chem Lett 24: 2777-80 (2014) Article DOI: 10.1016/j.bmcl.2014.03.069 BindingDB Entry DOI: 10.7270/Q2RV0Q8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50509061 (CHEMBL4554703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length DC-SIGN (unknown origin) expressed in human monocyte derived dendritic cells assessed as inhibition of binding to recombina... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50509063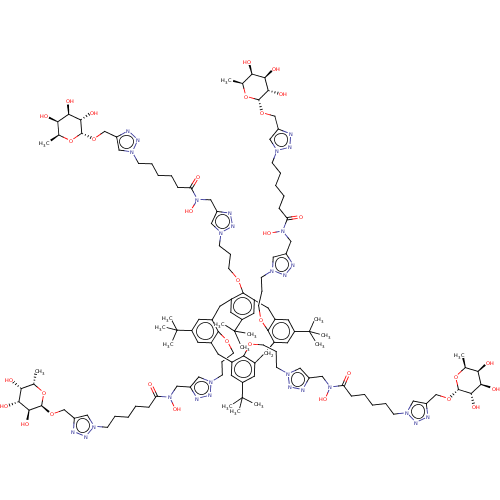 (CHEMBL4515649) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length DC-SIGN (unknown origin) expressed in human monocyte derived dendritic cells assessed as inhibition of binding to recombina... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50580861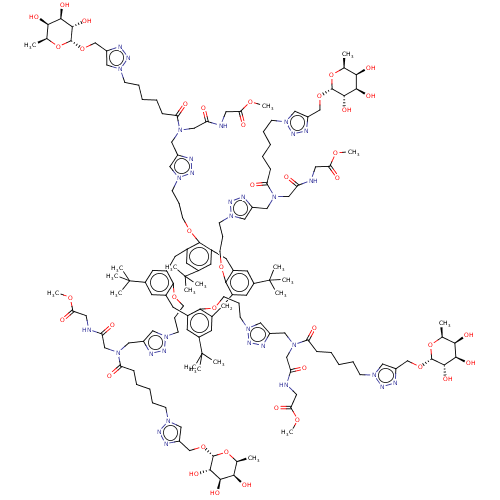 (CHEMBL5079779) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN (unknown origin) expressed in human Jurkat cells assessed as inhibition of EBOV-pseudotyped virus infection measured after 48 h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fucose-binding lectin PA-IIL (Pseudomonas aeruginosa) | BDBM50509063 (CHEMBL4515649) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 249 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universit£ de Picardie Jules Verne Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LecB by fluorescence polarization assay | J Med Chem 62: 7722-7738 (2019) Article DOI: 10.1021/acs.jmedchem.9b00481 BindingDB Entry DOI: 10.7270/Q2DB8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50509063 (CHEMBL4515649) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 289 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN (unknown origin) expressed in human Jurkat cells assessed as inhibition of EBOV-pseudotyped virus infection measured after 48 h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fucose-binding lectin PA-IIL (Pseudomonas aeruginosa) | BDBM50509060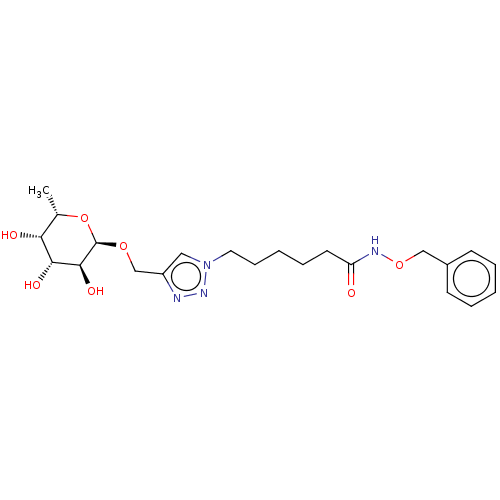 (CHEMBL4515315) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universit£ de Picardie Jules Verne Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LecB by fluorescence polarization assay | J Med Chem 62: 7722-7738 (2019) Article DOI: 10.1021/acs.jmedchem.9b00481 BindingDB Entry DOI: 10.7270/Q2DB8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50580864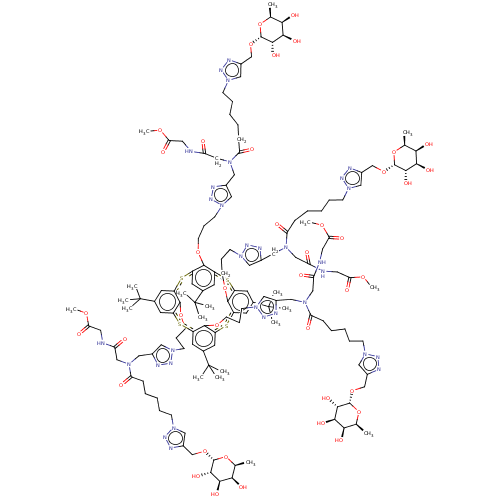 (CHEMBL5077032) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 602 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN (unknown origin) expressed in human Jurkat cells assessed as inhibition of EBOV-pseudotyped virus infection measured after 48 h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50509061 (CHEMBL4554703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 634 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN (unknown origin) expressed in human Jurkat cells assessed as inhibition of EBOV-pseudotyped virus infection measured after 48 h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fucose-binding lectin PA-IIL (Pseudomonas aeruginosa) | BDBM50509061 (CHEMBL4554703) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 664 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universit£ de Picardie Jules Verne Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LecB by fluorescence polarization assay | J Med Chem 62: 7722-7738 (2019) Article DOI: 10.1021/acs.jmedchem.9b00481 BindingDB Entry DOI: 10.7270/Q2DB8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50580861 (CHEMBL5079779) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 777 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length DC-SIGN (unknown origin) expressed in human monocyte derived dendritic cells assessed as inhibition of binding to recombina... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fucose-binding lectin PA-IIL (Pseudomonas aeruginosa) | BDBM50505617 (CHEMBL1234282) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universit£ de Picardie Jules Verne Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LecB by fluorescence polarization assay | J Med Chem 62: 7722-7738 (2019) Article DOI: 10.1021/acs.jmedchem.9b00481 BindingDB Entry DOI: 10.7270/Q2DB8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50580864 (CHEMBL5077032) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length DC-SIGN (unknown origin) expressed in human monocyte derived dendritic cells assessed as inhibition of binding to recombina... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fucose-binding lectin PA-IIL (Pseudomonas aeruginosa) | BDBM50242419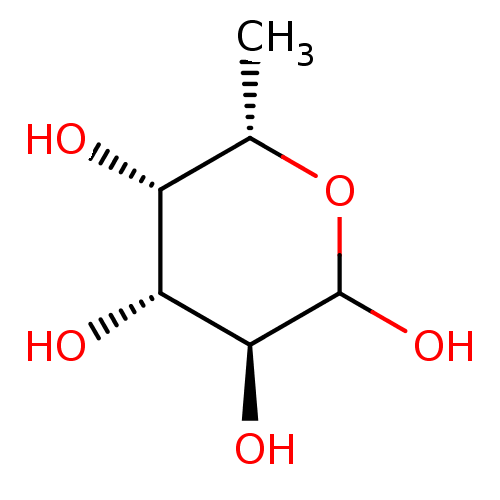 (6-Deoxy-L-galactose | 6-deoxy-L-galactopyranoseL-f...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universit£ de Picardie Jules Verne Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LecB by fluorescence polarization assay | J Med Chem 62: 7722-7738 (2019) Article DOI: 10.1021/acs.jmedchem.9b00481 BindingDB Entry DOI: 10.7270/Q2DB8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50580862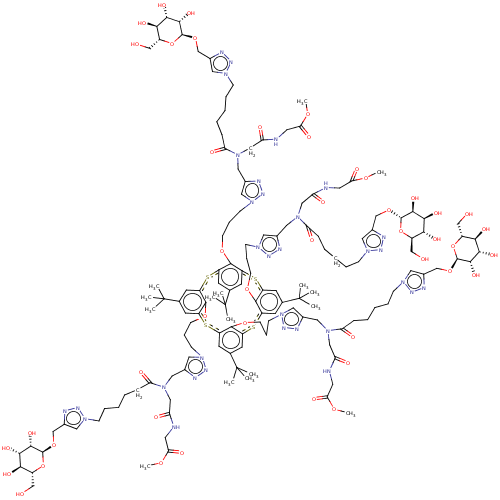 (CHEMBL5080461) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN extracellular domain (unknown origin) binding to HIV gp120 by surface plasmon resonance analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50580864 (CHEMBL5077032) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN extracellular domain (unknown origin) binding to HIV gp120 by surface plasmon resonance analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50580861 (CHEMBL5079779) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN extracellular domain (unknown origin) binding to HIV gp120 by surface plasmon resonance analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50509063 (CHEMBL4515649) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN extracellular domain (unknown origin) binding to HIV gp120 by surface plasmon resonance analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50509061 (CHEMBL4554703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN extracellular domain (unknown origin) binding to HIV gp120 by surface plasmon resonance analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50580863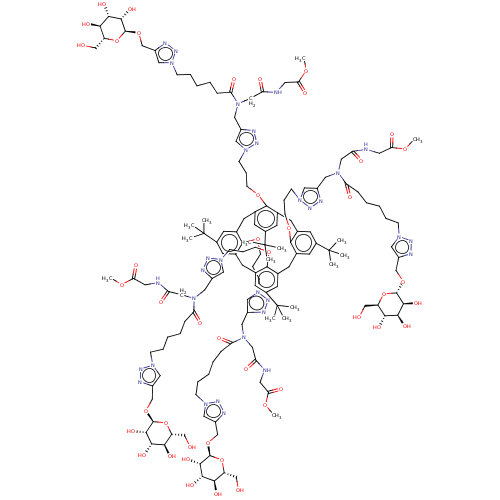 (CHEMBL5070715) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN extracellular domain (unknown origin) binding to HIV gp120 by surface plasmon resonance analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fucose-binding lectin PA-IIL (Pseudomonas aeruginosa) | BDBM50509062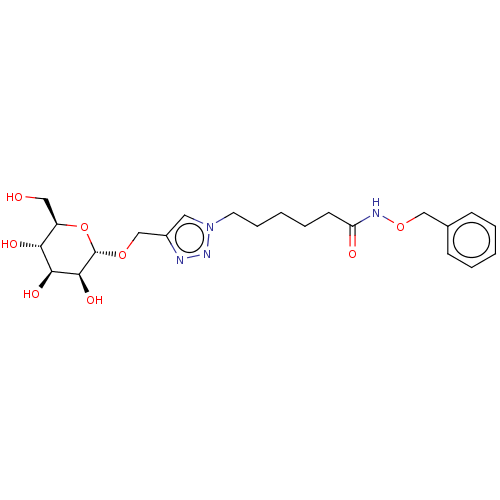 (CHEMBL4587299) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universit£ de Picardie Jules Verne Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LecB by fluorescence polarization assay | J Med Chem 62: 7722-7738 (2019) Article DOI: 10.1021/acs.jmedchem.9b00481 BindingDB Entry DOI: 10.7270/Q2DB8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fucose-binding lectin PA-IIL (Pseudomonas aeruginosa) | BDBM50166886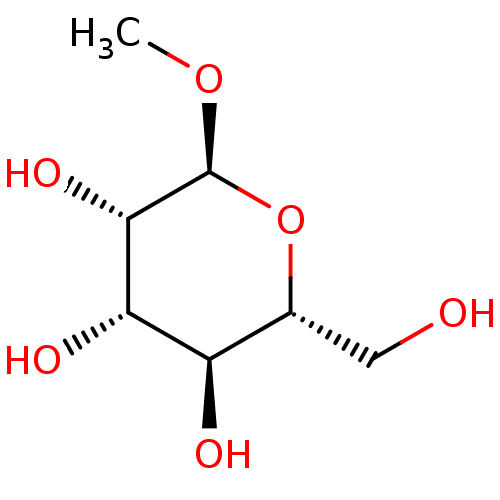 (CHEMBL195368 | methyl alpha-D-mannoside) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universit£ de Picardie Jules Verne Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LecB by fluorescence polarization assay | J Med Chem 62: 7722-7738 (2019) Article DOI: 10.1021/acs.jmedchem.9b00481 BindingDB Entry DOI: 10.7270/Q2DB8554 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50166886 (CHEMBL195368 | methyl alpha-D-mannoside) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.27E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN (unknown origin) expressed in human Jurkat cells assessed as inhibition of EBOV-pseudotyped virus infection measured after 48 h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50580865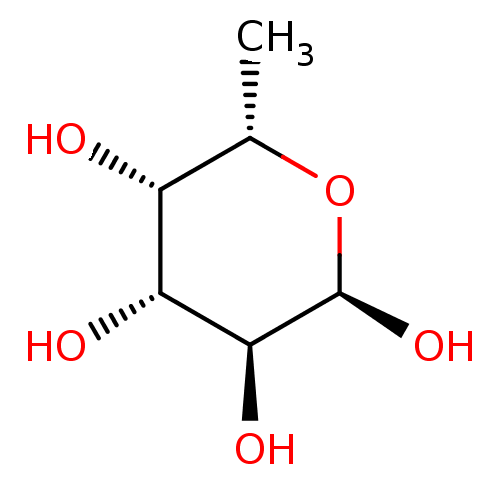 (CHEBI:42548 | CHEMBL1232862) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.48E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN extracellular domain (unknown origin) binding to HIV gp120 by surface plasmon resonance analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-type lectin domain family 4 member M (Homo sapiens) | BDBM50467903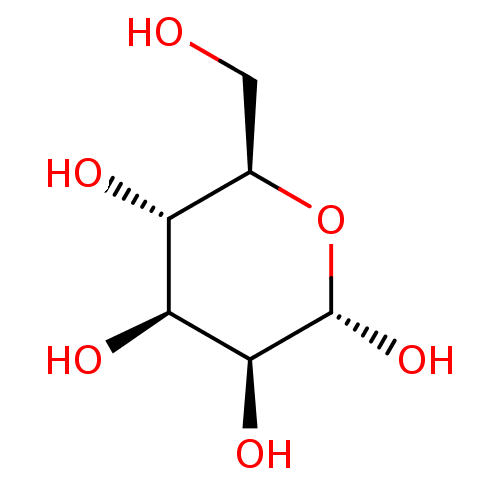 (CHEBI:28729 | CHEMBL365590) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.39E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of DC-SIGN extracellular domain (unknown origin) binding to HIV gp120 by surface plasmon resonance analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00818 BindingDB Entry DOI: 10.7270/Q2474FR1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||