 Found 86 hits with Last Name = 'benicchi' and Initial = 't'
Found 86 hits with Last Name = 'benicchi' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585934
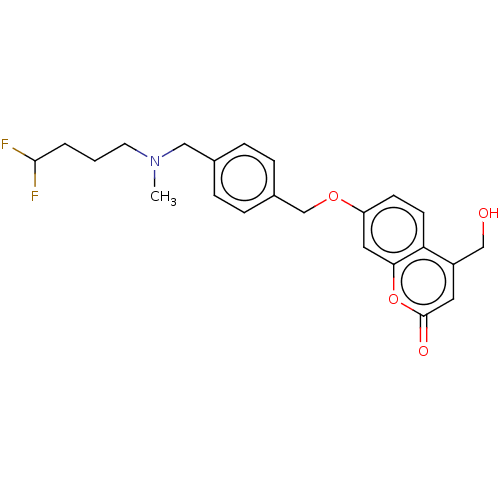
(CHEMBL5082824)Show SMILES CN(CCCC(F)F)Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human recombinant MAO-B expressed in supersomes using kynuramine as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585934

(CHEMBL5082824)Show SMILES CN(CCCC(F)F)Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of human AChE by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50441357

(CHEMBL2431805)Show InChI InChI=1S/C14H11F3N2OS/c15-14(16,17)9-3-1-8(2-4-9)12-18-11-7-21-6-5-10(11)13(20)19-12/h1-4H,5-7H2,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sienabiotech S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Tankyrases 2 (unknown origin) by autoPARsylationassay |
Eur J Med Chem 95: 526-45 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.055
BindingDB Entry DOI: 10.7270/Q28W3G1F |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585934

(CHEMBL5082824)Show SMILES CN(CCCC(F)F)Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585935

(CHEMBL5090153)Show SMILES CN(Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1)Cc1cccc(F)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585930

(CHEMBL3586771)Show SMILES CN(Cc1ccccc1)Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50441357

(CHEMBL2431805)Show InChI InChI=1S/C14H11F3N2OS/c15-14(16,17)9-3-1-8(2-4-9)12-18-11-7-21-6-5-10(11)13(20)19-12/h1-4H,5-7H2,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sienabiotech S.p.A.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Tankyrases 1 (unknown origin) by autoPARsylationassay |
Eur J Med Chem 95: 526-45 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.055
BindingDB Entry DOI: 10.7270/Q28W3G1F |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585923

(CHEMBL5083014)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCCN(Cc4cccc(F)c4)C3)ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM19187
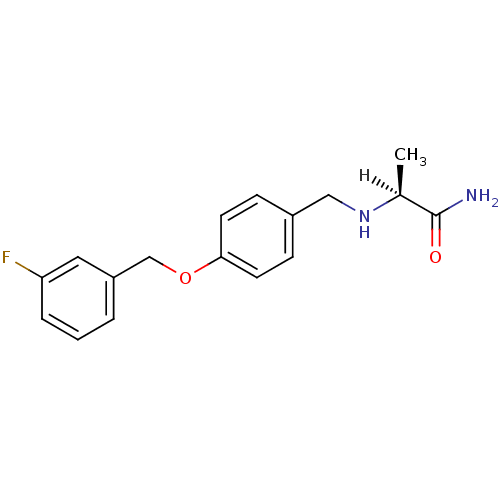
((2S)-2-[({4-[(3-fluorophenyl)methoxy]phenyl}methyl...)Show SMILES [H][C@@](C)(NCc1ccc(OCc2cccc(F)c2)cc1)C(N)=O |r| Show InChI InChI=1S/C17H19FN2O2/c1-12(17(19)21)20-10-13-5-7-16(8-6-13)22-11-14-3-2-4-15(18)9-14/h2-9,12,20H,10-11H2,1H3,(H2,19,21)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant CYP3A4 expressed in baculosomes by fluorescent homogenous assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50532731
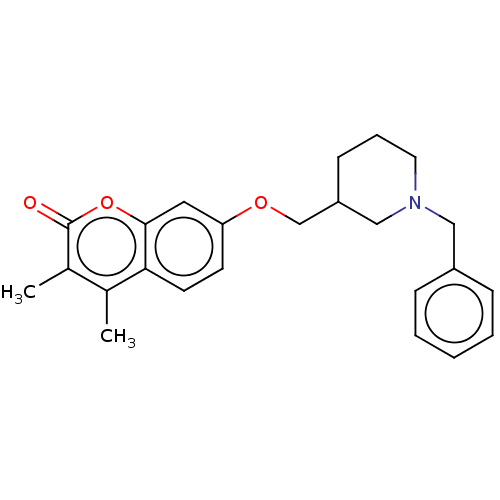
(CHEMBL4571763)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCCN(Cc4ccccc4)C3)ccc12 Show InChI InChI=1S/C24H27NO3/c1-17-18(2)24(26)28-23-13-21(10-11-22(17)23)27-16-20-9-6-12-25(15-20)14-19-7-4-3-5-8-19/h3-5,7-8,10-11,13,20H,6,9,12,14-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585927
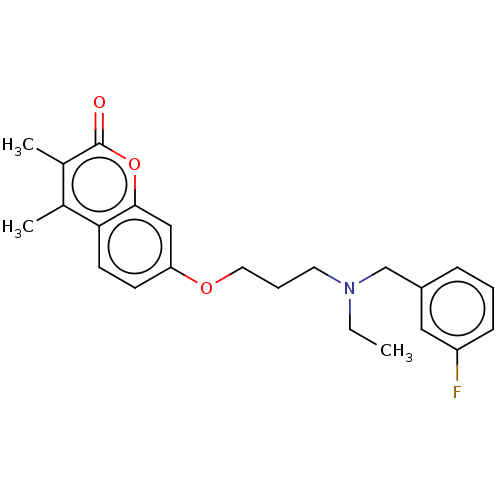
(CHEMBL5081663)Show SMILES CCN(CCCOc1ccc2c(C)c(C)c(=O)oc2c1)Cc1cccc(F)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585931
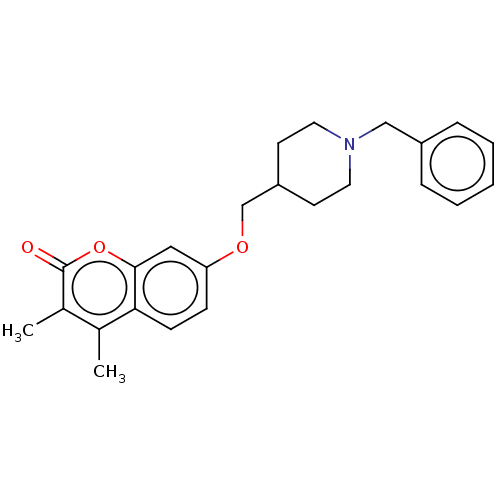
(CHEMBL4598256)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCN(Cc4ccccc4)CC3)ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585930

(CHEMBL3586771)Show SMILES CN(Cc1ccccc1)Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585926
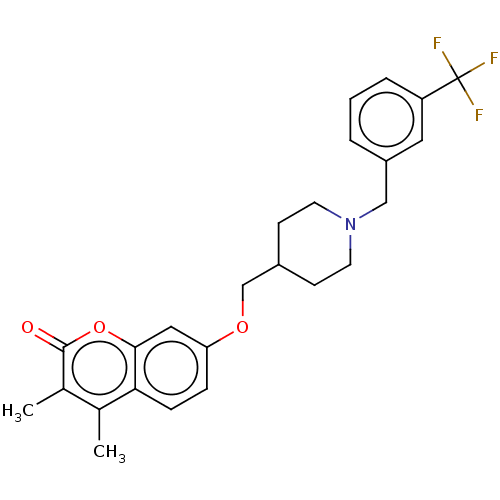
(CHEMBL5085674)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCN(Cc4cccc(c4)C(F)(F)F)CC3)ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585936
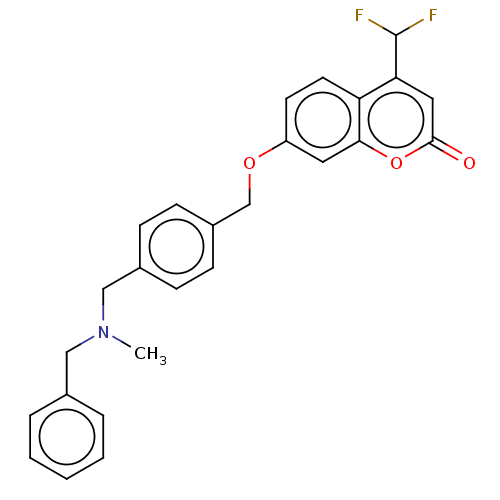
(CHEMBL5083613)Show SMILES CN(Cc1ccccc1)Cc1ccc(COc2ccc3c(cc(=O)oc3c2)C(F)F)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585925
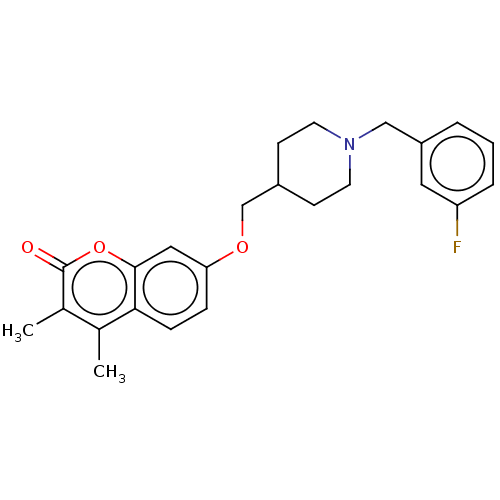
(CHEMBL5090504)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCN(Cc4cccc(F)c4)CC3)ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585933
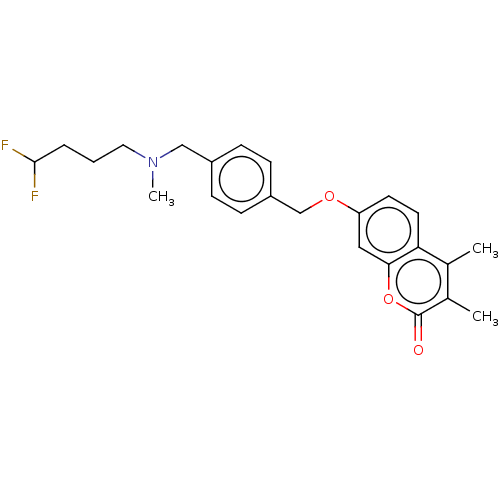
(CHEMBL5078658)Show SMILES CN(CCCC(F)F)Cc1ccc(COc2ccc3c(C)c(C)c(=O)oc3c2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585924
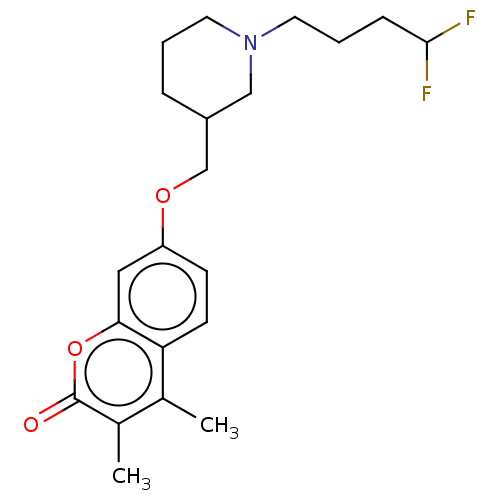
(CHEMBL5072382)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCCN(CCCC(F)F)C3)ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585935

(CHEMBL5090153)Show SMILES CN(Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1)Cc1cccc(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585928
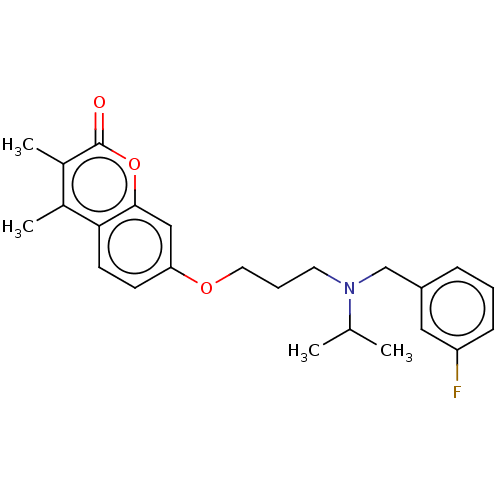
(CHEMBL5094348)Show SMILES CC(C)N(CCCOc1ccc2c(C)c(C)c(=O)oc2c1)Cc1cccc(F)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585931

(CHEMBL4598256)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCN(Cc4ccccc4)CC3)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585937
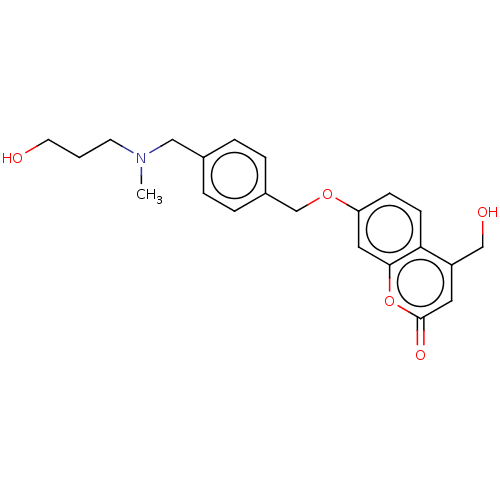
(CHEMBL5079544)Show SMILES CN(CCCO)Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50585936

(CHEMBL5083613)Show SMILES CN(Cc1ccccc1)Cc1ccc(COc2ccc3c(cc(=O)oc3c2)C(F)F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of horse serum BChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585925

(CHEMBL5090504)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCN(Cc4cccc(F)c4)CC3)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585926

(CHEMBL5085674)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCN(Cc4cccc(c4)C(F)(F)F)CC3)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585924

(CHEMBL5072382)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCCN(CCCC(F)F)C3)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50506808
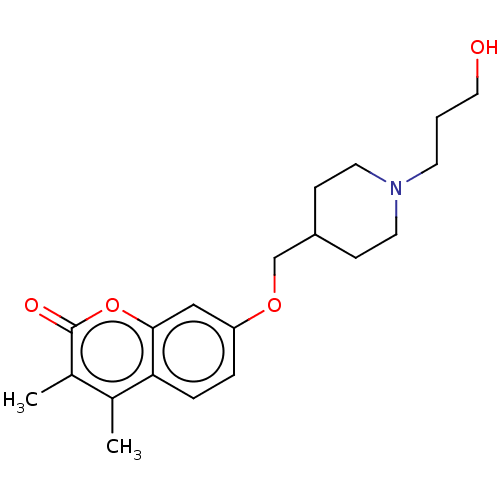
(CHEMBL4464759)Show InChI InChI=1S/C20H27NO4/c1-14-15(2)20(23)25-19-12-17(4-5-18(14)19)24-13-16-6-9-21(10-7-16)8-3-11-22/h4-5,12,16,22H,3,6-11,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585932
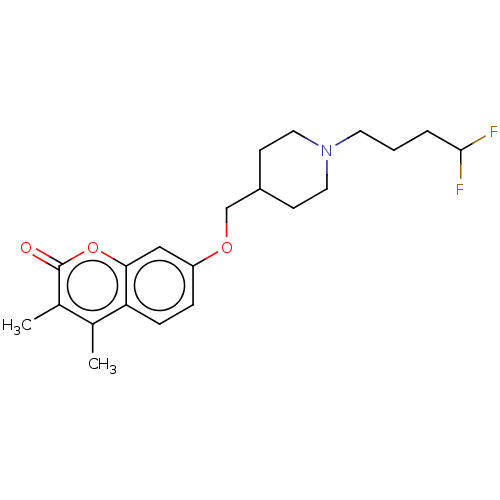
(CHEMBL5081574)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCN(CCCC(F)F)CC3)ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585934

(CHEMBL5082824)Show SMILES CN(CCCC(F)F)Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585936

(CHEMBL5083613)Show SMILES CN(Cc1ccccc1)Cc1ccc(COc2ccc3c(cc(=O)oc3c2)C(F)F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585929
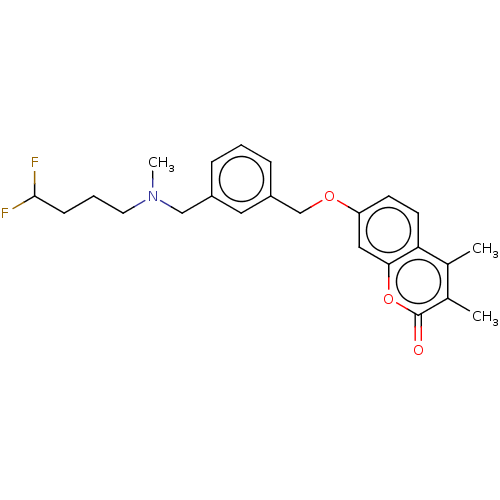
(CHEMBL5083737)Show SMILES CN(CCCC(F)F)Cc1cccc(COc2ccc3c(C)c(C)c(=O)oc3c2)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50585929

(CHEMBL5083737)Show SMILES CN(CCCC(F)F)Cc1cccc(COc2ccc3c(C)c(C)c(=O)oc3c2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant CYP3A4 expressed in baculosomes by fluorescent homogenous assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50585932

(CHEMBL5081574)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCN(CCCC(F)F)CC3)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant CYP3A4 expressed in baculosomes by fluorescent homogenous assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50532731

(CHEMBL4571763)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCCN(Cc4ccccc4)C3)ccc12 Show InChI InChI=1S/C24H27NO3/c1-17-18(2)24(26)28-23-13-21(10-11-22(17)23)27-16-20-9-6-12-25(15-20)14-19-7-4-3-5-8-19/h3-5,7-8,10-11,13,20H,6,9,12,14-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50506808

(CHEMBL4464759)Show InChI InChI=1S/C20H27NO4/c1-14-15(2)20(23)25-19-12-17(4-5-18(14)19)24-13-16-6-9-21(10-7-16)8-3-11-22/h4-5,12,16,22H,3,6-11,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585928

(CHEMBL5094348)Show SMILES CC(C)N(CCCOc1ccc2c(C)c(C)c(=O)oc2c1)Cc1cccc(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50585930

(CHEMBL3586771)Show SMILES CN(Cc1ccccc1)Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of horse serum BChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50585935

(CHEMBL5090153)Show SMILES CN(Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1)Cc1cccc(F)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of horse serum BChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50506810
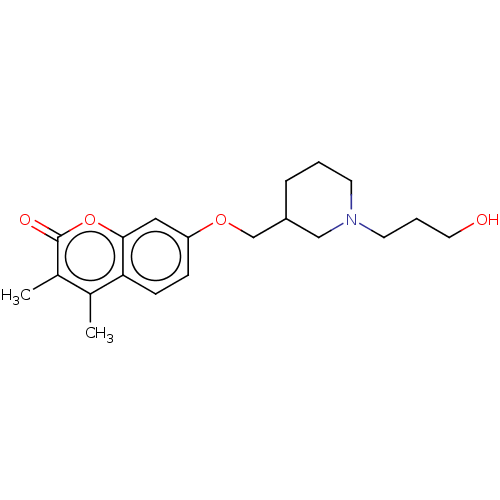
(CHEMBL4453856)Show InChI InChI=1S/C20H27NO4/c1-14-15(2)20(23)25-19-11-17(6-7-18(14)19)24-13-16-5-3-8-21(12-16)9-4-10-22/h6-7,11,16,22H,3-5,8-10,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585923

(CHEMBL5083014)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCCN(Cc4cccc(F)c4)C3)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585932

(CHEMBL5081574)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCN(CCCC(F)F)CC3)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585927

(CHEMBL5081663)Show SMILES CCN(CCCOc1ccc2c(C)c(C)c(=O)oc2c1)Cc1cccc(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50585926

(CHEMBL5085674)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCN(Cc4cccc(c4)C(F)(F)F)CC3)ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-A expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585933

(CHEMBL5078658)Show SMILES CN(CCCC(F)F)Cc1ccc(COc2ccc3c(C)c(C)c(=O)oc3c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50506810

(CHEMBL4453856)Show InChI InChI=1S/C20H27NO4/c1-14-15(2)20(23)25-19-11-17(6-7-18(14)19)24-13-16-5-3-8-21(12-16)9-4-10-22/h6-7,11,16,22H,3-5,8-10,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-B expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50585929

(CHEMBL5083737)Show SMILES CN(CCCC(F)F)Cc1cccc(COc2ccc3c(C)c(C)c(=O)oc3c2)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of horse serum BChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585929

(CHEMBL5083737)Show SMILES CN(CCCC(F)F)Cc1cccc(COc2ccc3c(C)c(C)c(=O)oc3c2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE by Ellman's spectrophotometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50585923

(CHEMBL5083014)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCCN(Cc4cccc(F)c4)C3)ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant MAO-A expressed in supersomes assessed as inhibition of 4-hydroxyquinoline formation using kynuramine as substrate by... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data