| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50585929 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2164321 (CHEMBL5049182) |
|---|
| IC50 | 600±n/a nM |
|---|
| Citation |  Rullo, M; Cipolloni, M; Catto, M; Colliva, C; Miniero, DV; Latronico, T; de Candia, M; Benicchi, T; Linusson, A; Giacchè, N; Altomare, CD; Pisani, L Probing Fluorinated Motifs onto Dual AChE-MAO B Inhibitors: Rational Design, Synthesis, Biological Evaluation, and Early-ADME Studies. J Med Chem65:3962-3977 (2022) [PubMed] Article Rullo, M; Cipolloni, M; Catto, M; Colliva, C; Miniero, DV; Latronico, T; de Candia, M; Benicchi, T; Linusson, A; Giacchè, N; Altomare, CD; Pisani, L Probing Fluorinated Motifs onto Dual AChE-MAO B Inhibitors: Rational Design, Synthesis, Biological Evaluation, and Early-ADME Studies. J Med Chem65:3962-3977 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50585929 |
|---|
| n/a |
|---|
| Name | BDBM50585929 |
|---|
| Synonyms: | CHEMBL5083737 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H27F2NO3 |
|---|
| Mol. Mass. | 415.4729 |
|---|
| SMILES | CN(CCCC(F)F)Cc1cccc(COc2ccc3c(C)c(C)c(=O)oc3c2)c1 |
|---|
| Structure | 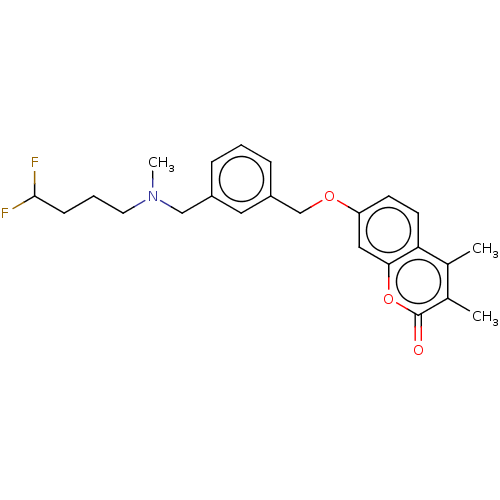
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Rullo, M; Cipolloni, M; Catto, M; Colliva, C; Miniero, DV; Latronico, T; de Candia, M; Benicchi, T; Linusson, A; Giacchè, N; Altomare, CD; Pisani, L Probing Fluorinated Motifs onto Dual AChE-MAO B Inhibitors: Rational Design, Synthesis, Biological Evaluation, and Early-ADME Studies. J Med Chem65:3962-3977 (2022) [PubMed] Article
Rullo, M; Cipolloni, M; Catto, M; Colliva, C; Miniero, DV; Latronico, T; de Candia, M; Benicchi, T; Linusson, A; Giacchè, N; Altomare, CD; Pisani, L Probing Fluorinated Motifs onto Dual AChE-MAO B Inhibitors: Rational Design, Synthesis, Biological Evaluation, and Early-ADME Studies. J Med Chem65:3962-3977 (2022) [PubMed] Article