 Found 2027 hits with Last Name = 'catto' and Initial = 'm'
Found 2027 hits with Last Name = 'catto' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21392

(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50019754
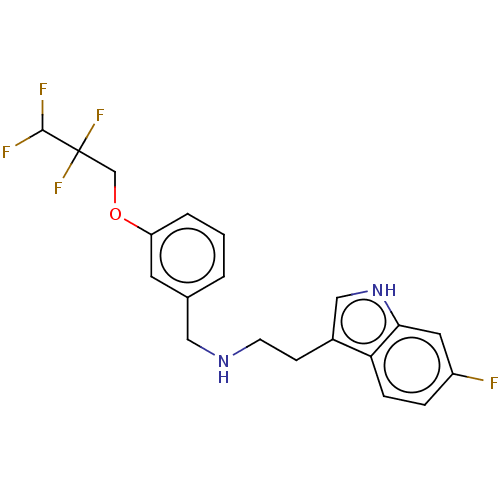
(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50219206
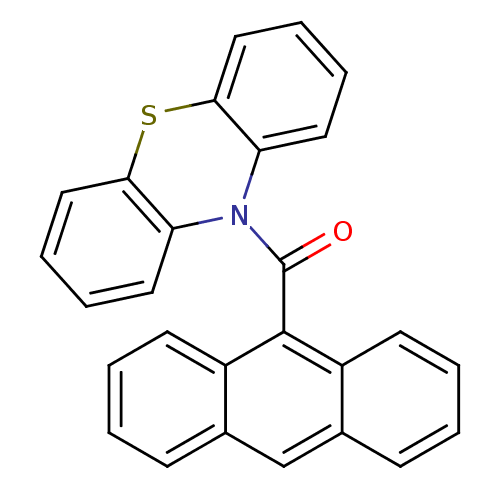
(Anthracen-9-yl (10H-phenothiazine-10yl) methanone,...)Show SMILES O=C(N1c2ccccc2Sc2ccccc12)c1c2ccccc2cc2ccccc12 Show InChI InChI=1S/C27H17NOS/c29-27(26-20-11-3-1-9-18(20)17-19-10-2-4-12-21(19)26)28-22-13-5-7-15-24(22)30-25-16-8-6-14-23(25)28/h1-17H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50219206

(Anthracen-9-yl (10H-phenothiazine-10yl) methanone,...)Show SMILES O=C(N1c2ccccc2Sc2ccccc12)c1c2ccccc2cc2ccccc12 Show InChI InChI=1S/C27H17NOS/c29-27(26-20-11-3-1-9-18(20)17-19-10-2-4-12-21(19)26)28-22-13-5-7-15-24(22)30-25-16-8-6-14-23(25)28/h1-17H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes BChE |
Eur J Med Chem 46: 2170-84 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.071
BindingDB Entry DOI: 10.7270/Q2BK1CPG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50402704

(CHEMBL2207369)Show InChI InChI=1S/C19H21N3O2S/c23-25(24,17-6-2-1-3-7-17)22-12-9-18-16(5-4-8-19(18)22)15-21-13-10-20-11-14-21/h1-9,12,20H,10-11,13-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50614136

(CHEMBL5287809) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50424045
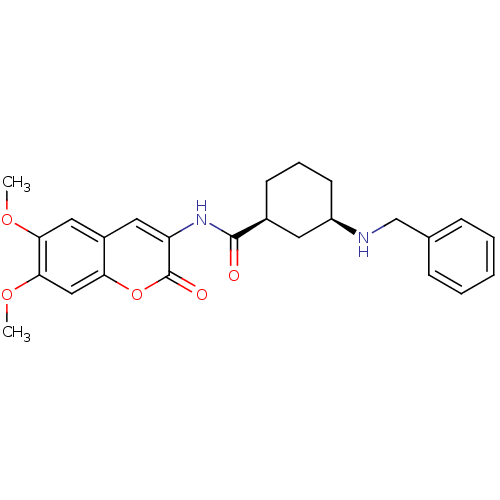
(CHEMBL2314726)Show SMILES COc1cc2cc(NC(=O)[C@H]3CCC[C@H](C3)NCc3ccccc3)c(=O)oc2cc1OC |r| Show InChI InChI=1S/C25H28N2O5/c1-30-22-13-18-12-20(25(29)32-21(18)14-23(22)31-2)27-24(28)17-9-6-10-19(11-17)26-15-16-7-4-3-5-8-16/h3-5,7-8,12-14,17,19,26H,6,9-11,15H2,1-2H3,(H,27,28)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Mixed-type reversible inhibition of bovine acetylcholinesterase using S-acetylthiocholine as substrate incubated for 20 mins prior to substrate addit... |
Bioorg Med Chem 21: 146-52 (2012)
Article DOI: 10.1016/j.bmc.2012.10.045
BindingDB Entry DOI: 10.7270/Q2QV3NTH |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50585934
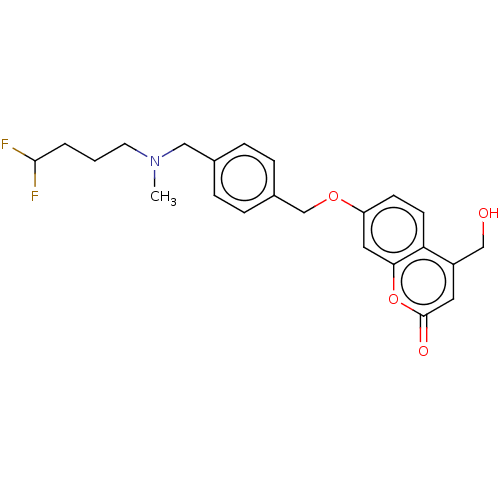
(CHEMBL5082824)Show SMILES CN(CCCC(F)F)Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human recombinant MAO-B expressed in supersomes using kynuramine as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method |
ACS Med Chem Lett 11: 869-876 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00656
BindingDB Entry DOI: 10.7270/Q2WS8XS4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50540764

(CHEMBL4648322)Show SMILES COc1cc2cc(NC(=O)[C@@H]3CCC[C@@H](C3)NCc3ccccc3)c(=O)oc2cc1OC |r| Show InChI InChI=1S/C25H28N2O5/c1-30-22-13-18-12-20(25(29)32-21(18)14-23(22)31-2)27-24(28)17-9-6-10-19(11-17)26-15-16-7-4-3-5-8-16/h3-5,7-8,12-14,17,19,26H,6,9-11,15H2,1-2H3,(H,27,28)/t17-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method |
ACS Med Chem Lett 11: 869-876 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00656
BindingDB Entry DOI: 10.7270/Q2WS8XS4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50342853

(4-(6,7-Dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)-...)Show SMILES COc1cc2CC[N+](Cc2cc1OC)=c1ccn(Cc2ccc(Cn3ccc(cc3)=[N+]3CCCCC3)cc2)cc1 |(22.64,-11.65,;21.3,-10.89,;19.97,-11.67,;18.64,-10.91,;17.31,-11.67,;15.97,-10.91,;14.64,-11.68,;14.66,-13.22,;15.99,-13.99,;17.31,-13.21,;18.64,-13.98,;19.97,-13.21,;21.31,-13.98,;22.64,-13.2,;13.33,-14,;13.34,-15.54,;12.01,-16.32,;10.67,-15.55,;9.34,-16.33,;8,-15.57,;6.67,-16.34,;5.33,-15.58,;5.33,-14.04,;4,-13.27,;2.66,-14.05,;2.67,-15.6,;1.33,-16.37,;-0,-15.6,;-0,-14.06,;1.33,-13.29,;-1.34,-16.37,;-2.67,-15.59,;-4,-16.35,;-4.01,-17.9,;-2.67,-18.67,;-1.33,-17.91,;6.65,-13.26,;7.99,-14.02,;10.66,-14.02,;11.98,-13.24,)| Show InChI InChI=1S/C34H40N4O2/c1-39-33-22-29-10-21-38(26-30(29)23-34(33)40-2)32-13-19-36(20-14-32)25-28-8-6-27(7-9-28)24-35-17-11-31(12-18-35)37-15-4-3-5-16-37/h6-9,11-14,17-20,22-23H,3-5,10,15-16,21,24-26H2,1-2H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of bovine AChE at 30 nM using S-acetylthiocholine as as substrate by Lineweaver-Burk plot analysis |
J Med Chem 54: 2627-45 (2011)
Article DOI: 10.1021/jm101299d
BindingDB Entry DOI: 10.7270/Q2SQ90QT |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50197240
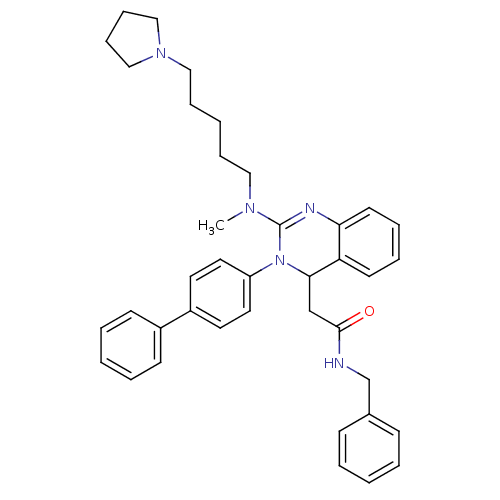
(CHEMBL248088 | KYS-05080 | N-Benzyl-2-{3-biphenyl-...)Show SMILES CN(CCCCCN1CCCC1)C1=Nc2ccccc2C(CC(=O)NCc2ccccc2)N1c1ccc(cc1)-c1ccccc1 |t:13| Show InChI InChI=1S/C39H45N5O/c1-42(25-11-4-12-26-43-27-13-14-28-43)39-41-36-20-10-9-19-35(36)37(29-38(45)40-30-31-15-5-2-6-16-31)44(39)34-23-21-33(22-24-34)32-17-7-3-8-18-32/h2-3,5-10,15-24,37H,4,11-14,25-30H2,1H3,(H,40,45) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Mixed/noncompetitive inhibition of equine serum BCHE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addi... |
Bioorg Med Chem Lett 27: 1179-1185 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.068
BindingDB Entry DOI: 10.7270/Q2P271CH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50222222
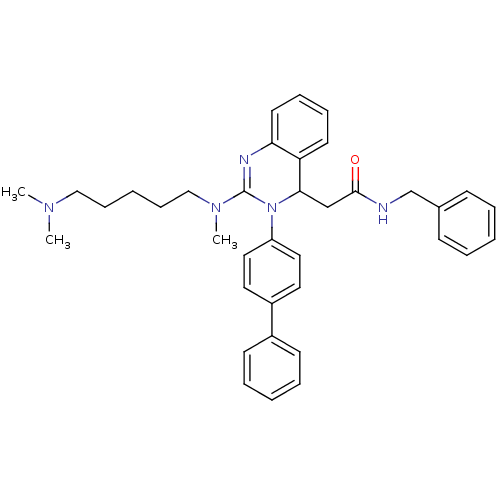
(CHEMBL394956 | KYS-05090 | N-benzyl-2-(3-(biphenyl...)Show SMILES CN(C)CCCCCN(C)C1=Nc2ccccc2C(CC(=O)NCc2ccccc2)N1c1ccc(cc1)-c1ccccc1 |t:10| Show InChI InChI=1S/C37H43N5O/c1-40(2)25-13-6-14-26-41(3)37-39-34-20-12-11-19-33(34)35(27-36(43)38-28-29-15-7-4-8-16-29)42(37)32-23-21-31(22-24-32)30-17-9-5-10-18-30/h4-5,7-12,15-24,35H,6,13-14,25-28H2,1-3H3,(H,38,43) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Mixed/noncompetitive inhibition of equine serum BCHE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addi... |
Bioorg Med Chem Lett 27: 1179-1185 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.068
BindingDB Entry DOI: 10.7270/Q2P271CH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50614134

(CHEMBL5275931) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50614142
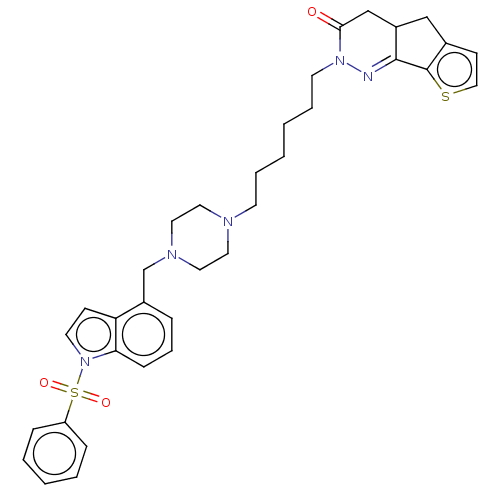
(CHEMBL5285645) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50093085
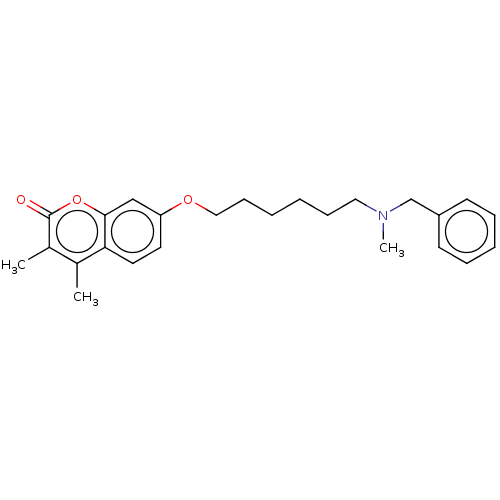
(CHEMBL3586582)Show SMILES CN(CCCCCCOc1ccc2c(C)c(C)c(=O)oc2c1)Cc1ccccc1 Show InChI InChI=1S/C25H31NO3/c1-19-20(2)25(27)29-24-17-22(13-14-23(19)24)28-16-10-5-4-9-15-26(3)18-21-11-7-6-8-12-21/h6-8,11-14,17H,4-5,9-10,15-16,18H2,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro"
Curated by ChEMBL
| Assay Description
Mixed type inhibition of electric eel AChE by Lineweaver-Burk plot |
J Med Chem 58: 5561-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00599
BindingDB Entry DOI: 10.7270/Q26T0PCZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50614137
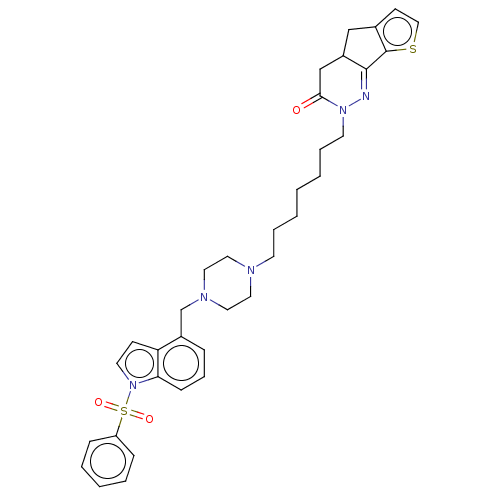
(CHEMBL5281673) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50614144

(CHEMBL5282158) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50424045

(CHEMBL2314726)Show SMILES COc1cc2cc(NC(=O)[C@H]3CCC[C@H](C3)NCc3ccccc3)c(=O)oc2cc1OC |r| Show InChI InChI=1S/C25H28N2O5/c1-30-22-13-18-12-20(25(29)32-21(18)14-23(22)31-2)27-24(28)17-9-6-10-19(11-17)26-15-16-7-4-3-5-8-16/h3-5,7-8,12-14,17,19,26H,6,9-11,15H2,1-2H3,(H,27,28)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method |
ACS Med Chem Lett 11: 869-876 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00656
BindingDB Entry DOI: 10.7270/Q2WS8XS4 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50532731
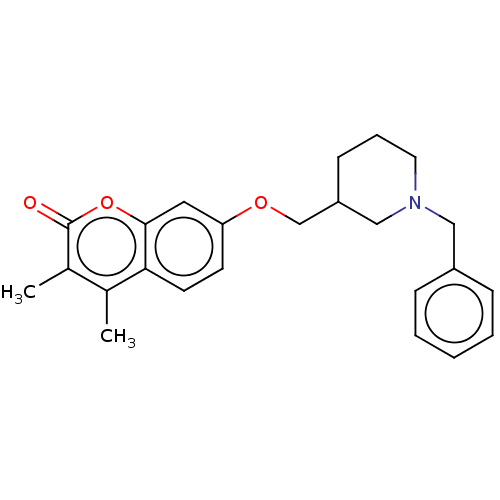
(CHEMBL4571763)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCCN(Cc4ccccc4)C3)ccc12 Show InChI InChI=1S/C24H27NO3/c1-17-18(2)24(26)28-23-13-21(10-11-22(17)23)27-16-20-9-6-12-25(15-20)14-19-7-4-3-5-8-19/h3-5,7-8,10-11,13,20H,6,9,12,14-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00001
BindingDB Entry DOI: 10.7270/Q2R49VSW |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50424045

(CHEMBL2314726)Show SMILES COc1cc2cc(NC(=O)[C@H]3CCC[C@H](C3)NCc3ccccc3)c(=O)oc2cc1OC |r| Show InChI InChI=1S/C25H28N2O5/c1-30-22-13-18-12-20(25(29)32-21(18)14-23(22)31-2)27-24(28)17-9-6-10-19(11-17)26-15-16-7-4-3-5-8-16/h3-5,7-8,12-14,17,19,26H,6,9-11,15H2,1-2H3,(H,27,28)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method |
ACS Med Chem Lett 11: 869-876 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00656
BindingDB Entry DOI: 10.7270/Q2WS8XS4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50524749
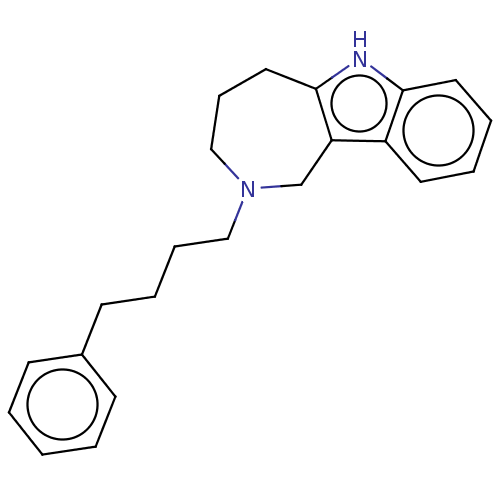
(CHEMBL4538834)Show InChI InChI=1S/C22H26N2/c1-2-9-18(10-3-1)11-6-7-15-24-16-8-14-22-20(17-24)19-12-4-5-13-21(19)23-22/h1-5,9-10,12-13,23H,6-8,11,14-17H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro
Curated by ChEMBL
| Assay Description
Mixed type inhibition of equine serum BChE assessed as Ki using butyrylthiocholine as substrate preincubated for 20 mins followed by substrate additi... |
Eur J Med Chem 177: 414-424 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.062
BindingDB Entry DOI: 10.7270/Q2RF5ZGP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50093227
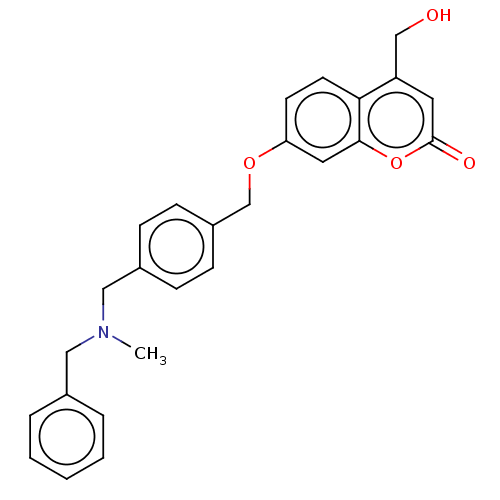
(CHEMBL3586608)Show SMILES Cl.CN(Cc1ccccc1)Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1 Show InChI InChI=1S/C17H13NO/c1-11-15-4-2-3-13-7-10-16(18(13)15)17(11)12-5-8-14(19)9-6-12/h2-10,19H,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro"
Curated by ChEMBL
| Assay Description
Mixed type inhibition of electric eel AChE by Lineweaver-Burk plot |
J Med Chem 58: 5561-78 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00599
BindingDB Entry DOI: 10.7270/Q26T0PCZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50614143
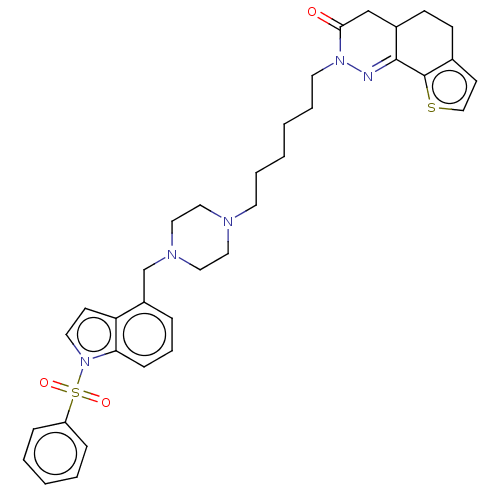
(CHEMBL5283896) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50614138
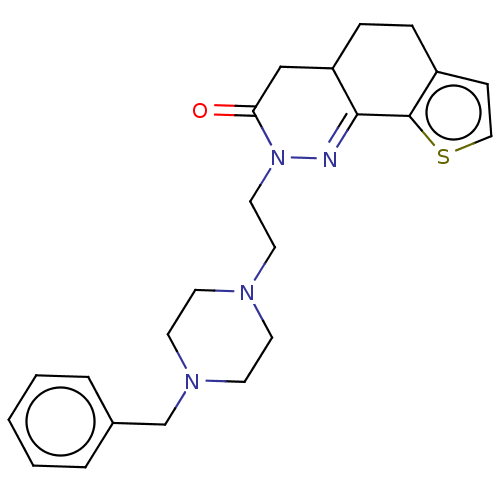
(CHEMBL5283494) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50532731

(CHEMBL4571763)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCCN(Cc4ccccc4)C3)ccc12 Show InChI InChI=1S/C24H27NO3/c1-17-18(2)24(26)28-23-13-21(10-11-22(17)23)27-16-20-9-6-12-25(15-20)14-19-7-4-3-5-8-19/h3-5,7-8,10-11,13,20H,6,9,12,14-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00001
BindingDB Entry DOI: 10.7270/Q2R49VSW |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50532731

(CHEMBL4571763)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCCN(Cc4ccccc4)C3)ccc12 Show InChI InChI=1S/C24H27NO3/c1-17-18(2)24(26)28-23-13-21(10-11-22(17)23)27-16-20-9-6-12-25(15-20)14-19-7-4-3-5-8-19/h3-5,7-8,10-11,13,20H,6,9,12,14-16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00001
BindingDB Entry DOI: 10.7270/Q2R49VSW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50614135
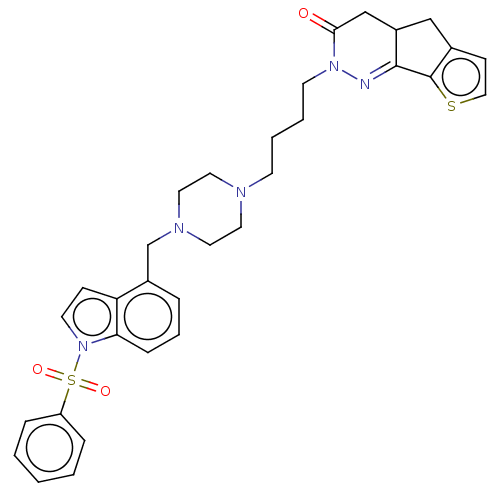
(CHEMBL5275394) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50614133

(CHEMBL5284727) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50614132
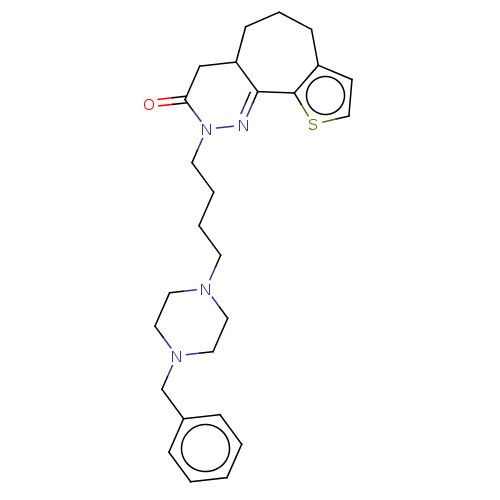
(CHEMBL5285633) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 414 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50614129
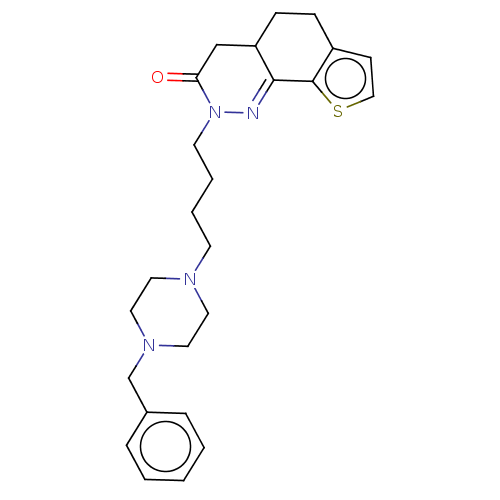
(CHEMBL5265814) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 496 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50614131
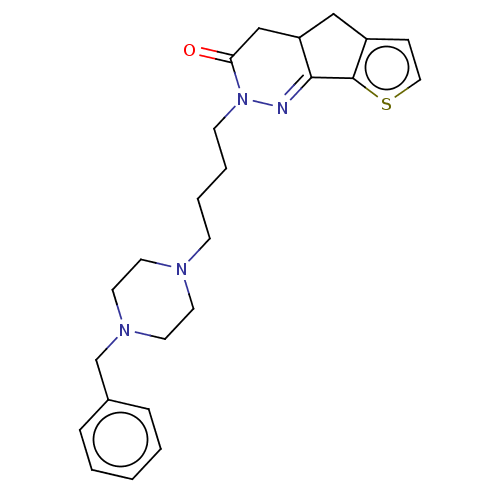
(CHEMBL5282788) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 614 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50614130
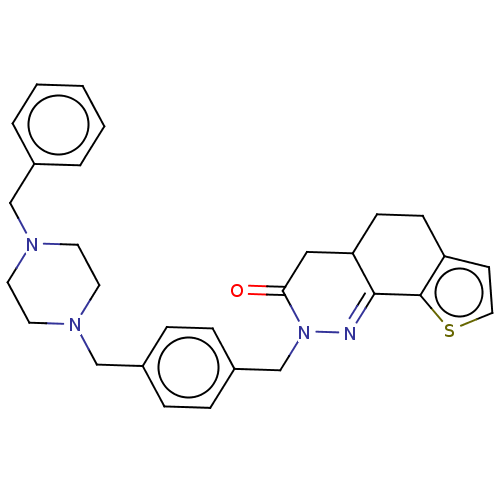
(CHEMBL5283557) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 786 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50614132

(CHEMBL5285633) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 976 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50614129

(CHEMBL5265814) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 995 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50614131

(CHEMBL5282788) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50614138

(CHEMBL5283494) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50614130

(CHEMBL5283557) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50532731

(CHEMBL4571763)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCCN(Cc4ccccc4)C3)ccc12 Show InChI InChI=1S/C24H27NO3/c1-17-18(2)24(26)28-23-13-21(10-11-22(17)23)27-16-20-9-6-12-25(15-20)14-19-7-4-3-5-8-19/h3-5,7-8,10-11,13,20H,6,9,12,14-16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro"
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AChE using S-acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition measured fo... |
J Med Chem 59: 6791-806 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00562
BindingDB Entry DOI: 10.7270/Q25142Q8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50532731

(CHEMBL4571763)Show SMILES Cc1c(C)c(=O)oc2cc(OCC3CCCN(Cc4ccccc4)C3)ccc12 Show InChI InChI=1S/C24H27NO3/c1-17-18(2)24(26)28-23-13-21(10-11-22(17)23)27-16-20-9-6-12-25(15-20)14-19-7-4-3-5-8-19/h3-5,7-8,10-11,13,20H,6,9,12,14-16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro"
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AChE using S-acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition measured fo... |
J Med Chem 59: 6791-806 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00562
BindingDB Entry DOI: 10.7270/Q25142Q8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50614132

(CHEMBL5285633) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50614138

(CHEMBL5283494) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50614134

(CHEMBL5275931) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50614136

(CHEMBL5287809) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50585934

(CHEMBL5082824)Show SMILES CN(CCCC(F)F)Cc1ccc(COc2ccc3c(CO)cc(=O)oc3c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of human AChE by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01784
BindingDB Entry DOI: 10.7270/Q2BG2SWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50614133

(CHEMBL5284727) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50614135

(CHEMBL5275394) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data