| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholinesterase |
|---|
| Ligand | BDBM50197240 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1648347 (CHEMBL3997403) |
|---|
| Ki | 29±n/a nM |
|---|
| Citation |  Park, B; Nam, JH; Kim, JH; Kim, HJ; Onnis, V; Balboni, G; Lee, KT; Park, JH; Catto, M; Carotti, A; Lee, JY 3,4-Dihydroquinazoline derivatives inhibit the activities of cholinesterase enzymes. Bioorg Med Chem Lett27:1179-1185 (2017) [PubMed] Article Park, B; Nam, JH; Kim, JH; Kim, HJ; Onnis, V; Balboni, G; Lee, KT; Park, JH; Catto, M; Carotti, A; Lee, JY 3,4-Dihydroquinazoline derivatives inhibit the activities of cholinesterase enzymes. Bioorg Med Chem Lett27:1179-1185 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholinesterase |
|---|
| Name: | Cholinesterase |
|---|
| Synonyms: | BCHE | Butyrylcholinesterase (BuChE) | CHLE_HORSE | Cholinesterase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 65643.35 |
|---|
| Organism: | Equus caballus (Horse) |
|---|
| Description: | P81908 |
|---|
| Residue: | 574 |
|---|
| Sequence: | EEDIIITTKNGKVRGMNLPVLGGTVTAFLGIPYAQPPLGRLRFKKPQSLTKWSNIWNATK
YANSCYQNTDQSFPGFLGSEMWNPNTELSEDCLYLNVWIPAPKPKNATVMIWIYGGGFQT
GTSSLPVYDGKFLARVERVIVVSMNYRVGALGFLALSENPEAPGNMGLFDQQLALQWVQK
NIAAFGGNPRSVTLFGESAGAASVSLHLLSPRSQPLFTRAILQSGSSNAPWAVTSLYEAR
NRTLTLAKRMGCSRDNETEMIKCLRDKDPQEILLNEVFVVPYDTLLSVNFGPTVDGDFLT
DMPDTLLQLGQFKRTQILVGVNKDEGTAFLVYGAPGFSKDNNSIITRKEFQEGLKIFFPR
VSEFGRESILFHYMDWLDDQRAENYREALDDVVGDYNIICPALEFTRKFSELGNDAFFYY
FEHRSTKLPWPEWMGVMHGYEIEFVFGLPLERRVNYTRAEEILSRSIMKRWANFAKYGNP
NGTQNNSTRWPVFKSTEQKYLTLNTESPKVYTKLRAQQCRFWTLFFPKVLELTGNIDEAE
REWKAGFHRWNNYMMDWKNQFNDYTSKKESCSDF
|
|
|
|---|
| BDBM50197240 |
|---|
| n/a |
|---|
| Name | BDBM50197240 |
|---|
| Synonyms: | CHEMBL248088 | KYS-05080 | N-Benzyl-2-{3-biphenyl-4-yl-2-[methyl-(5-pyrrolidin-1-yl-pentyl)-amino]-3,4-dihydro-quinazolin-4-yl}-acetamide | N-benzyl-2-(3-(biphenyl-4-yl)-2-(methyl(5-(pyrrolidin-1-yl)pentyl)amino)-3,4-dihydroquinazolin-4-yl)acetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C39H45N5O |
|---|
| Mol. Mass. | 599.8075 |
|---|
| SMILES | CN(CCCCCN1CCCC1)C1=Nc2ccccc2C(CC(=O)NCc2ccccc2)N1c1ccc(cc1)-c1ccccc1 |t:13| |
|---|
| Structure | 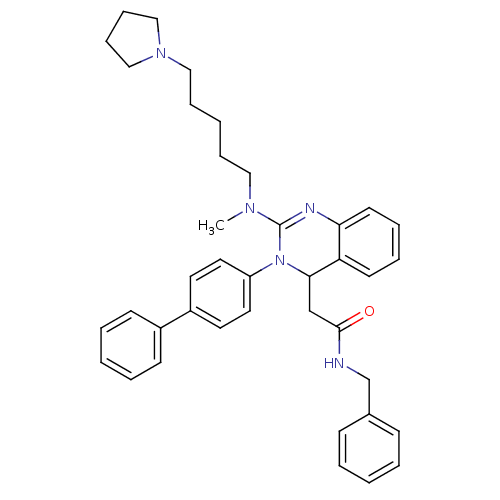
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Park, B; Nam, JH; Kim, JH; Kim, HJ; Onnis, V; Balboni, G; Lee, KT; Park, JH; Catto, M; Carotti, A; Lee, JY 3,4-Dihydroquinazoline derivatives inhibit the activities of cholinesterase enzymes. Bioorg Med Chem Lett27:1179-1185 (2017) [PubMed] Article
Park, B; Nam, JH; Kim, JH; Kim, HJ; Onnis, V; Balboni, G; Lee, KT; Park, JH; Catto, M; Carotti, A; Lee, JY 3,4-Dihydroquinazoline derivatives inhibit the activities of cholinesterase enzymes. Bioorg Med Chem Lett27:1179-1185 (2017) [PubMed] Article