 Found 676 hits with Last Name = 'lee' and Initial = 'kt'
Found 676 hits with Last Name = 'lee' and Initial = 'kt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Equus caballus (Horse)) | BDBM50197240
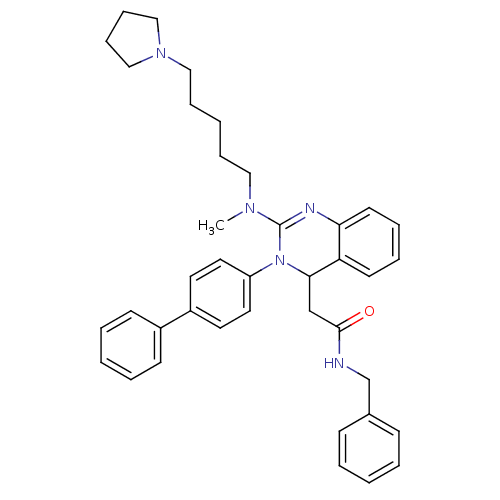
(CHEMBL248088 | KYS-05080 | N-Benzyl-2-{3-biphenyl-...)Show SMILES CN(CCCCCN1CCCC1)C1=Nc2ccccc2C(CC(=O)NCc2ccccc2)N1c1ccc(cc1)-c1ccccc1 |t:13| Show InChI InChI=1S/C39H45N5O/c1-42(25-11-4-12-26-43-27-13-14-28-43)39-41-36-20-10-9-19-35(36)37(29-38(45)40-30-31-15-5-2-6-16-31)44(39)34-23-21-33(22-24-34)32-17-7-3-8-18-32/h2-3,5-10,15-24,37H,4,11-14,25-30H2,1H3,(H,40,45) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Mixed/noncompetitive inhibition of equine serum BCHE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addi... |
Bioorg Med Chem Lett 27: 1179-1185 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.068
BindingDB Entry DOI: 10.7270/Q2P271CH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50222222
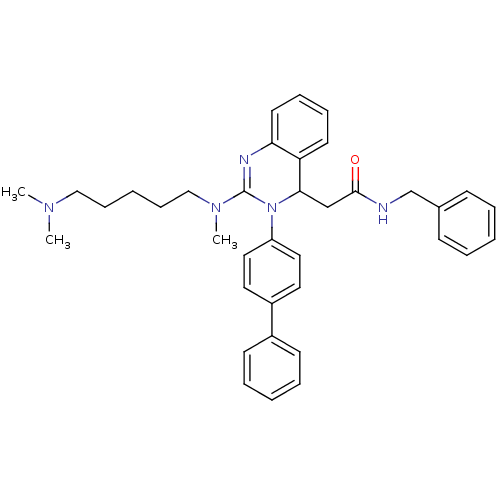
(CHEMBL394956 | KYS-05090 | N-benzyl-2-(3-(biphenyl...)Show SMILES CN(C)CCCCCN(C)C1=Nc2ccccc2C(CC(=O)NCc2ccccc2)N1c1ccc(cc1)-c1ccccc1 |t:10| Show InChI InChI=1S/C37H43N5O/c1-40(2)25-13-6-14-26-41(3)37-39-34-20-12-11-19-33(34)35(27-36(43)38-28-29-15-7-4-8-16-29)42(37)32-23-21-31(22-24-32)30-17-9-5-10-18-30/h4-5,7-12,15-24,35H,6,13-14,25-28H2,1-3H3,(H,38,43) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Mixed/noncompetitive inhibition of equine serum BCHE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addi... |
Bioorg Med Chem Lett 27: 1179-1185 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.068
BindingDB Entry DOI: 10.7270/Q2P271CH |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 2
(Homo sapiens (Human)) | BDBM50134199
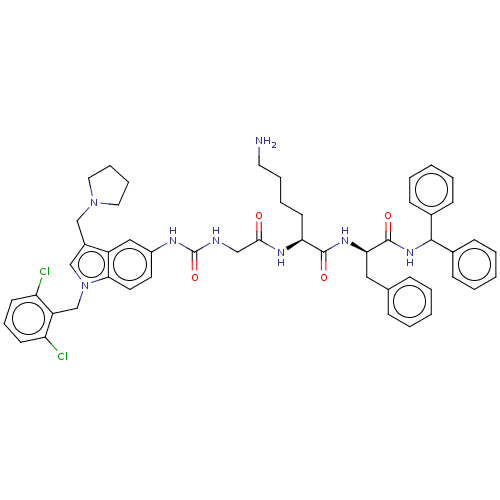
(CHEMBL3735057)Show SMILES NCCCC[C@H](NC(=O)CNC(=O)Nc1ccc2n(Cc3c(Cl)cccc3Cl)cc(CN3CCCC3)c2c1)C(=O)N[C@H](Cc1ccccc1)C(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C51H56Cl2N8O4/c52-42-21-14-22-43(53)41(42)34-61-33-38(32-60-27-12-13-28-60)40-30-39(24-25-46(40)61)56-51(65)55-31-47(62)57-44(23-10-11-26-54)49(63)58-45(29-35-15-4-1-5-16-35)50(64)59-48(36-17-6-2-7-18-36)37-19-8-3-9-20-37/h1-9,14-22,24-25,30,33,44-45,48H,10-13,23,26-29,31-32,34,54H2,(H,57,62)(H,58,63)(H,59,64)(H2,55,56,65)/t44-,45+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 627 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]furoyl-LIGRL-NH2 from human protease-activated receptor 2 in NCTC-2544 cells |
Bioorg Med Chem 23: 7717-27 (2015)
Article DOI: 10.1016/j.bmc.2015.11.016
BindingDB Entry DOI: 10.7270/Q2VH5QP3 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 2
(Homo sapiens (Human)) | BDBM50134200
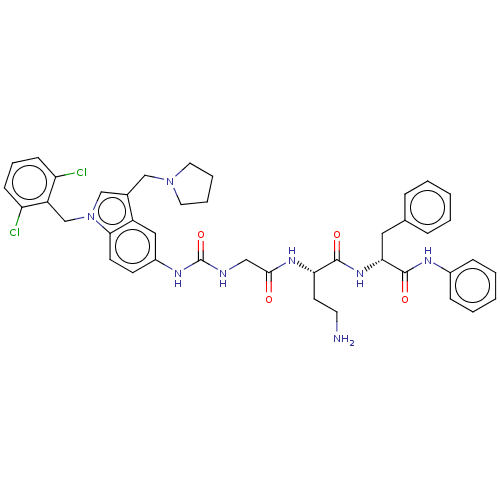
(CHEMBL3735405)Show SMILES NCC[C@H](NC(=O)CNC(=O)Nc1ccc2n(Cc3c(Cl)cccc3Cl)cc(CN3CCCC3)c2c1)C(=O)N[C@H](Cc1ccccc1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C42H46Cl2N8O4/c43-34-14-9-15-35(44)33(34)27-52-26-29(25-51-20-7-8-21-51)32-23-31(16-17-38(32)52)48-42(56)46-24-39(53)49-36(18-19-45)40(54)50-37(22-28-10-3-1-4-11-28)41(55)47-30-12-5-2-6-13-30/h1-6,9-17,23,26,36-37H,7-8,18-22,24-25,27,45H2,(H,47,55)(H,49,53)(H,50,54)(H2,46,48,56)/t36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]furoyl-LIGRL-NH2 from human protease-activated receptor 2 in NCTC-2544 cells |
Bioorg Med Chem 23: 7717-27 (2015)
Article DOI: 10.1016/j.bmc.2015.11.016
BindingDB Entry DOI: 10.7270/Q2VH5QP3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50585092
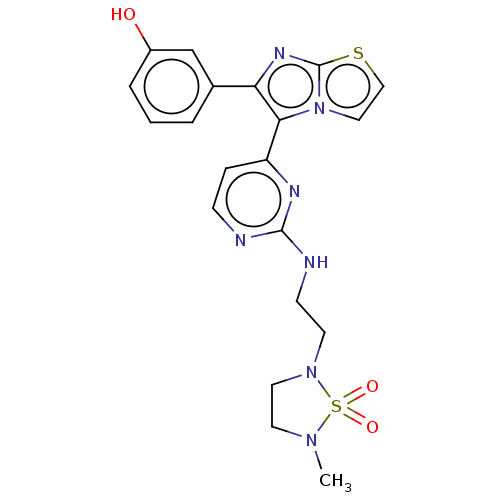
(CHEMBL5087577)Show SMILES CN1CCN(CCNc2nccc(n2)-c2c(nc3sccn23)-c2cccc(O)c2)S1(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00230
BindingDB Entry DOI: 10.7270/Q2RV0SMN |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CAMK2b (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of RSK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Homeodomain-interacting protein kinase 1
(Homo sapiens (Human)) | BDBM50400734
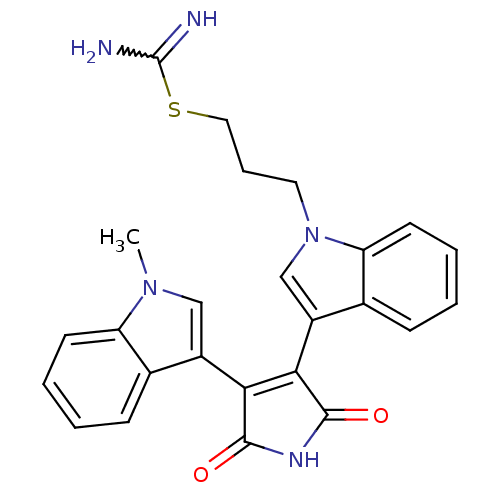
(CHEMBL1591531)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(CCCSC(N)=N)c3ccccc23)c2ccccc12 |w:18.19,t:4| Show InChI InChI=1S/C25H23N5O2S/c1-29-13-17(15-7-2-4-9-19(15)29)21-22(24(32)28-23(21)31)18-14-30(11-6-12-33-25(26)27)20-10-5-3-8-16(18)20/h2-5,7-10,13-14H,6,11-12H2,1H3,(H3,26,27)(H,28,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.367 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of HIPK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50364573
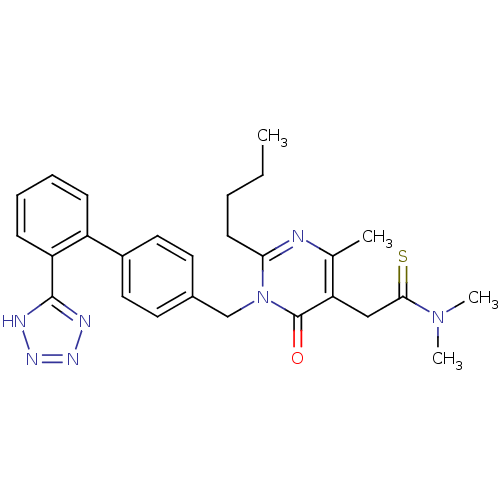
(CHEMBL1951143)Show SMILES CCCCc1nc(C)c(CC(=S)N(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C27H31N7OS/c1-5-6-11-24-28-18(2)23(16-25(36)33(3)4)27(35)34(24)17-19-12-14-20(15-13-19)21-9-7-8-10-22(21)26-29-31-32-30-26/h7-10,12-15H,5-6,11,16-17H2,1-4H3,(H,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 receptor type 1 in New Zealand White rabbit descending thoracic aorta rings assessed as inhibition of angiotensi... |
Bioorg Med Chem Lett 22: 1649-54 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.116
BindingDB Entry DOI: 10.7270/Q22R3S48 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Misshapen-like kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of MINK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.473 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of LYN (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Phosphorylase b kinase gamma catalytic chain, liver/testis isoform
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PHKg2 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50428286

(DABRAFENIB | GSK2118436A)Show SMILES CC(C)(C)c1nc(c(s1)-c1ccnc(N)n1)-c1cccc(NS(=O)(=O)c2c(F)cccc2F)c1F Show InChI InChI=1S/C23H20F3N5O2S2/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25/h4-11,31H,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113277
BindingDB Entry DOI: 10.7270/Q2VX0M8Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin A (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50585081

(CHEMBL5086749)Show SMILES Oc1cccc(c1)-c1nc2sccn2c1-c1ccnc(NCCCNS(=O)(=O)c2cccc(F)c2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00230
BindingDB Entry DOI: 10.7270/Q2RV0SMN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) after 120 mins in presence of 33P-ATP |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM435721

(US10570155, Compound 25III | US11332479, Compound ...)Show SMILES Oc1cccc(c1)-c1nc2sccn2c1-c1ccnc(NCCNS(=O)(=O)c2ccc(F)cc2)n1 Show InChI InChI=1S/C23H19FN6O3S2/c24-16-4-6-18(7-5-16)35(32,33)27-11-10-26-22-25-9-8-19(28-22)21-20(15-2-1-3-17(31)14-15)29-23-30(21)12-13-34-23/h1-9,12-14,27,31H,10-11H2,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00230
BindingDB Entry DOI: 10.7270/Q2RV0SMN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50358430
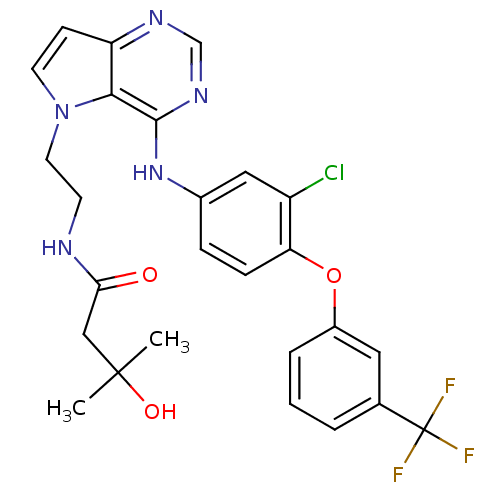
(CHEMBL1614725)Show SMILES CC(C)(O)CC(=O)NCCn1ccc2ncnc(Nc3ccc(Oc4cccc(c4)C(F)(F)F)c(Cl)c3)c12 Show InChI InChI=1S/C26H25ClF3N5O3/c1-25(2,37)14-22(36)31-9-11-35-10-8-20-23(35)24(33-15-32-20)34-17-6-7-21(19(27)13-17)38-18-5-3-4-16(12-18)26(28,29)30/h3-8,10,12-13,15,37H,9,11,14H2,1-2H3,(H,31,36)(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-diprenorphine binding to kappa opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50364587
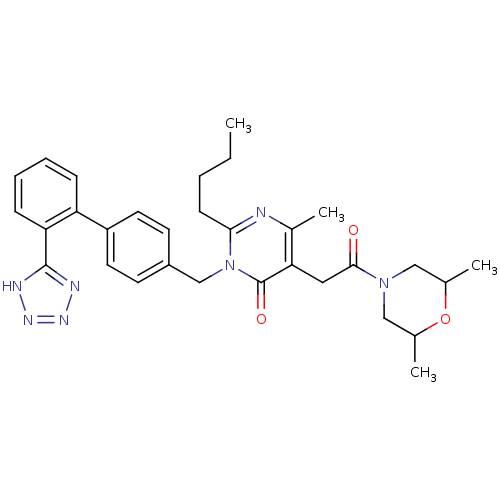
(CHEMBL1951136)Show SMILES CCCCc1nc(C)c(CC(=O)N2CC(C)OC(C)C2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H37N7O3/c1-5-6-11-28-32-22(4)27(16-29(39)37-17-20(2)41-21(3)18-37)31(40)38(28)19-23-12-14-24(15-13-23)25-9-7-8-10-26(25)30-33-35-36-34-30/h7-10,12-15,20-21H,5-6,11,16-19H2,1-4H3,(H,33,34,35,36) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 receptor type 1 in New Zealand White rabbit descending thoracic aorta rings assessed as inhibition of angiotensi... |
Bioorg Med Chem Lett 22: 1649-54 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.116
BindingDB Entry DOI: 10.7270/Q22R3S48 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50585092

(CHEMBL5087577)Show SMILES CN1CCN(CCNc2nccc(n2)-c2c(nc3sccn23)-c2cccc(O)c2)S1(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human wild type CRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00230
BindingDB Entry DOI: 10.7270/Q2RV0SMN |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50569443
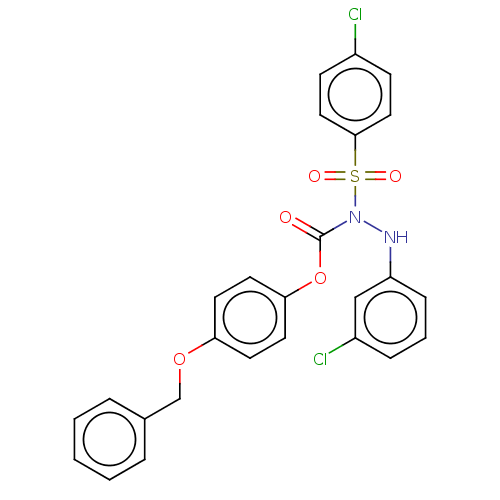
(CHEMBL4851179)Show SMILES Clc1ccc(cc1)S(=O)(=O)N(Nc1cccc(Cl)c1)C(=O)Oc1ccc(OCc2ccccc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128920
BindingDB Entry DOI: 10.7270/Q23B6438 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50364574
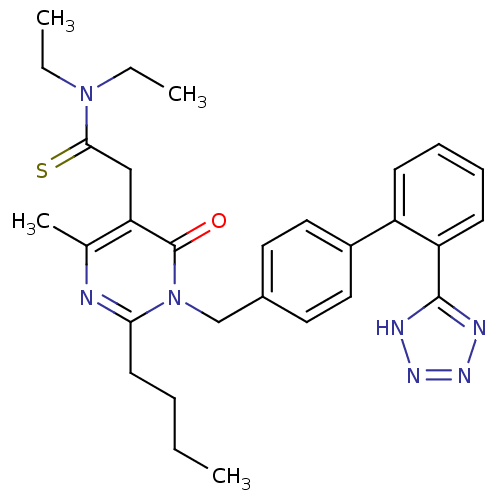
(CHEMBL1951144)Show SMILES CCCCc1nc(C)c(CC(=S)N(CC)CC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H35N7OS/c1-5-8-13-26-30-20(4)25(18-27(38)35(6-2)7-3)29(37)36(26)19-21-14-16-22(17-15-21)23-11-9-10-12-24(23)28-31-33-34-32-28/h9-12,14-17H,5-8,13,18-19H2,1-4H3,(H,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 receptor type 1 in New Zealand White rabbit descending thoracic aorta rings assessed as inhibition of angiotensi... |
Bioorg Med Chem Lett 22: 1649-54 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.116
BindingDB Entry DOI: 10.7270/Q22R3S48 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50585085

(CHEMBL5080553)Show SMILES COc1cccc(c1)-c1nc2sccn2c1-c1ccnc(NCCN2CCN(C)S2(=O)=O)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00230
BindingDB Entry DOI: 10.7270/Q2RV0SMN |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50364580
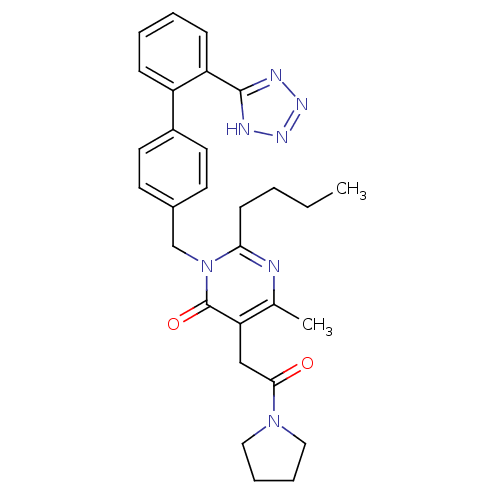
(CHEMBL1951129)Show SMILES CCCCc1nc(C)c(CC(=O)N2CCCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H33N7O2/c1-3-4-11-26-30-20(2)25(18-27(37)35-16-7-8-17-35)29(38)36(26)19-21-12-14-22(15-13-21)23-9-5-6-10-24(23)28-31-33-34-32-28/h5-6,9-10,12-15H,3-4,7-8,11,16-19H2,1-2H3,(H,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 receptor type 1 in New Zealand White rabbit descending thoracic aorta rings assessed as inhibition of angiotensi... |
Bioorg Med Chem Lett 22: 1649-54 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.116
BindingDB Entry DOI: 10.7270/Q22R3S48 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/cyclin B (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of RET (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50364586
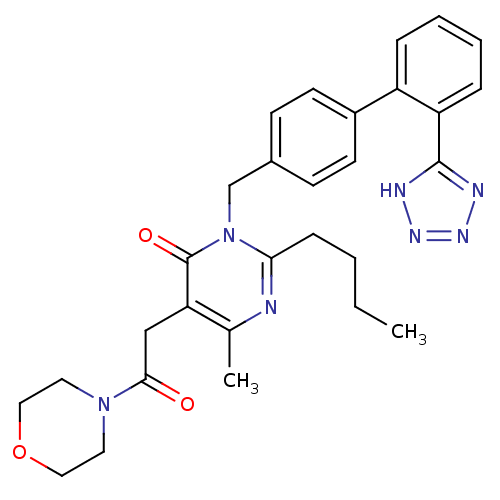
(CHEMBL1951135)Show SMILES CCCCc1nc(C)c(CC(=O)N2CCOCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H33N7O3/c1-3-4-9-26-30-20(2)25(18-27(37)35-14-16-39-17-15-35)29(38)36(26)19-21-10-12-22(13-11-21)23-7-5-6-8-24(23)28-31-33-34-32-28/h5-8,10-13H,3-4,9,14-19H2,1-2H3,(H,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 receptor type 1 in New Zealand White rabbit descending thoracic aorta rings assessed as inhibition of angiotensi... |
Bioorg Med Chem Lett 22: 1649-54 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.116
BindingDB Entry DOI: 10.7270/Q22R3S48 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50585095
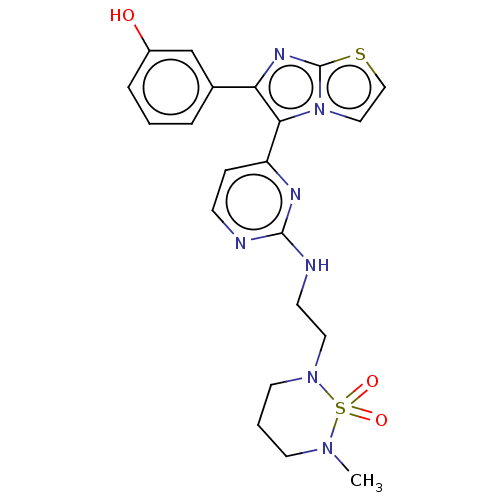
(CHEMBL5079413)Show SMILES CN1CCCN(CCNc2nccc(n2)-c2c(nc3sccn23)-c2cccc(O)c2)S1(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human wild type CRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00230
BindingDB Entry DOI: 10.7270/Q2RV0SMN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50585092

(CHEMBL5087577)Show SMILES CN1CCN(CCNc2nccc(n2)-c2c(nc3sccn23)-c2cccc(O)c2)S1(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human wild type BRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00230
BindingDB Entry DOI: 10.7270/Q2RV0SMN |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50364581
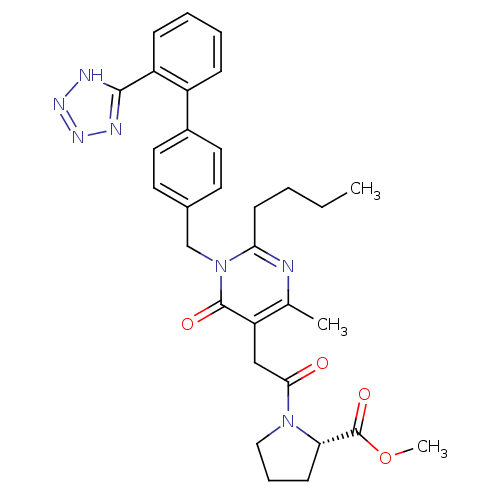
(CHEMBL1951130)Show SMILES CCCCc1nc(C)c(CC(=O)N2CCC[C@H]2C(=O)OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C31H35N7O4/c1-4-5-12-27-32-20(2)25(18-28(39)37-17-8-11-26(37)31(41)42-3)30(40)38(27)19-21-13-15-22(16-14-21)23-9-6-7-10-24(23)29-33-35-36-34-29/h6-7,9-10,13-16,26H,4-5,8,11-12,17-19H2,1-3H3,(H,33,34,35,36)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.06 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 receptor type 1 in New Zealand White rabbit descending thoracic aorta rings assessed as inhibition of angiotensi... |
Bioorg Med Chem Lett 22: 1649-54 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.116
BindingDB Entry DOI: 10.7270/Q22R3S48 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50364582
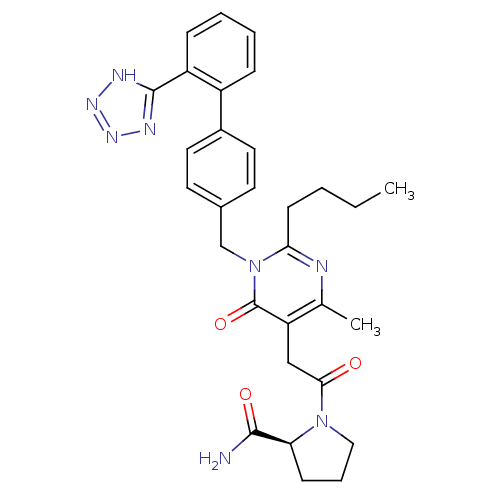
(CHEMBL1951131)Show SMILES CCCCc1nc(C)c(CC(=O)N2CCC[C@H]2C(N)=O)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C30H34N8O3/c1-3-4-11-26-32-19(2)24(17-27(39)37-16-7-10-25(37)28(31)40)30(41)38(26)18-20-12-14-21(15-13-20)22-8-5-6-9-23(22)29-33-35-36-34-29/h5-6,8-9,12-15,25H,3-4,7,10-11,16-18H2,1-2H3,(H2,31,40)(H,33,34,35,36)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 receptor type 1 in New Zealand White rabbit descending thoracic aorta rings assessed as inhibition of angiotensi... |
Bioorg Med Chem Lett 22: 1649-54 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.116
BindingDB Entry DOI: 10.7270/Q22R3S48 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G1/S-specific cyclin- 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/cyclin E (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of c-SRC (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50585088
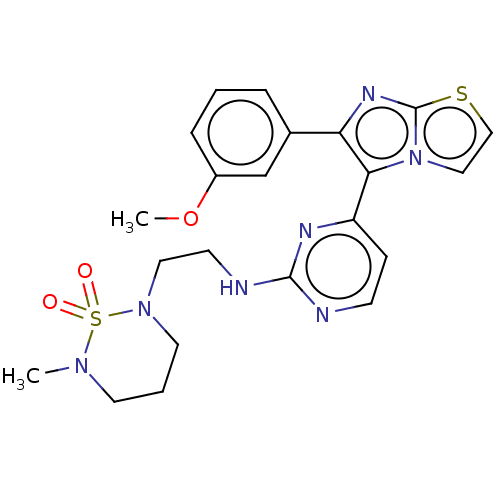
(CHEMBL5073246)Show SMILES COc1cccc(c1)-c1nc2sccn2c1-c1ccnc(NCCN2CCCN(C)S2(=O)=O)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human wild type CRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00230
BindingDB Entry DOI: 10.7270/Q2RV0SMN |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR3 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50364577
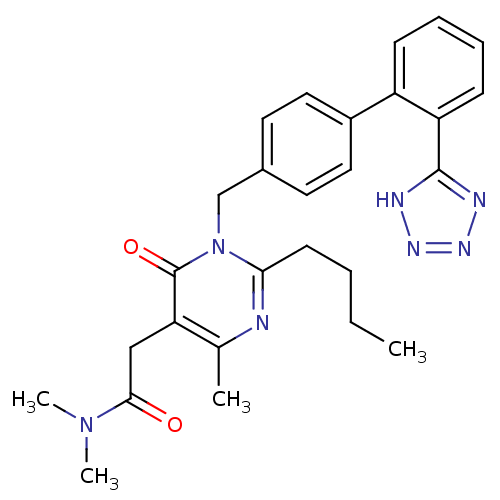
(CHEMBL1951126)Show SMILES CCCCc1nc(C)c(CC(=O)N(C)C)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C27H31N7O2/c1-5-6-11-24-28-18(2)23(16-25(35)33(3)4)27(36)34(24)17-19-12-14-20(15-13-19)21-9-7-8-10-22(21)26-29-31-32-30-26/h7-10,12-15H,5-6,11,16-17H2,1-4H3,(H,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.37 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 receptor type 1 in New Zealand White rabbit descending thoracic aorta rings assessed as inhibition of angiotensi... |
Bioorg Med Chem Lett 22: 1649-54 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.116
BindingDB Entry DOI: 10.7270/Q22R3S48 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50364583
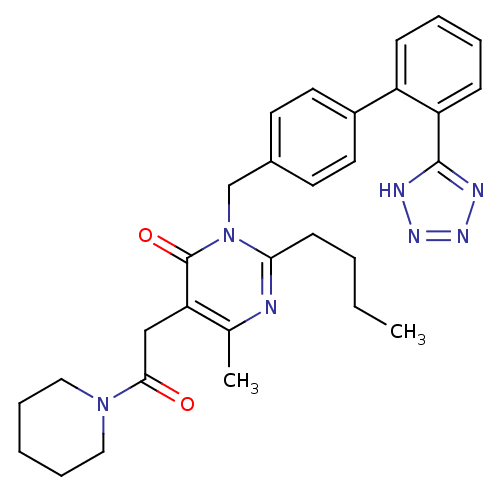
(CHEMBL1951132)Show SMILES CCCCc1nc(C)c(CC(=O)N2CCCCC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H35N7O2/c1-3-4-12-27-31-21(2)26(19-28(38)36-17-8-5-9-18-36)30(39)37(27)20-22-13-15-23(16-14-22)24-10-6-7-11-25(24)29-32-34-35-33-29/h6-7,10-11,13-16H,3-5,8-9,12,17-20H2,1-2H3,(H,32,33,34,35) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.46 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 receptor type 1 in New Zealand White rabbit descending thoracic aorta rings assessed as inhibition of angiotensi... |
Bioorg Med Chem Lett 22: 1649-54 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.116
BindingDB Entry DOI: 10.7270/Q22R3S48 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50563431
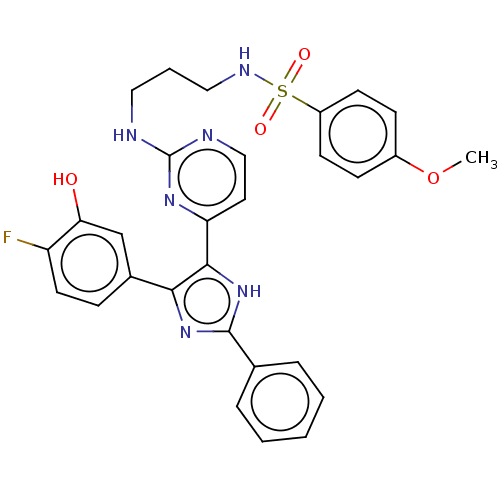
(CHEMBL4793723)Show SMILES COc1ccc(cc1)S(=O)(=O)NCCCNc1nccc(n1)-c1[nH]c(nc1-c1ccc(F)c(O)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) using Ser/Thr 03 as substrate after 1 hr in presence of ATP by Z'-LYTE assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113277
BindingDB Entry DOI: 10.7270/Q2VX0M8Q |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50585085

(CHEMBL5080553)Show SMILES COc1cccc(c1)-c1nc2sccn2c1-c1ccnc(NCCN2CCN(C)S2(=O)=O)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human wild type CRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00230
BindingDB Entry DOI: 10.7270/Q2RV0SMN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data