

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419871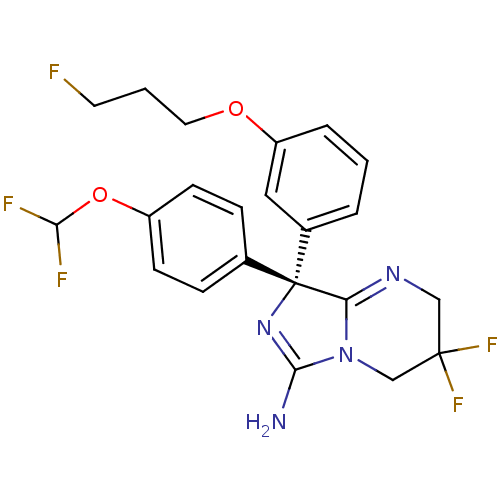 (CHEMBL1957483) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419864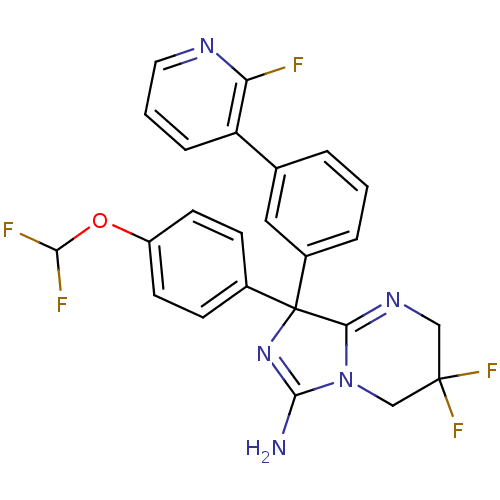 (CHEMBL1957476) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419858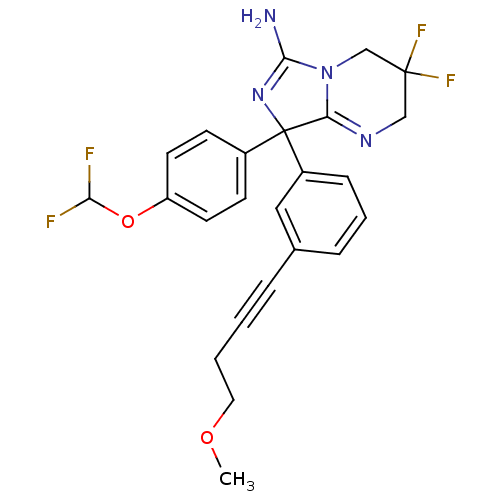 (CHEMBL1957481) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419863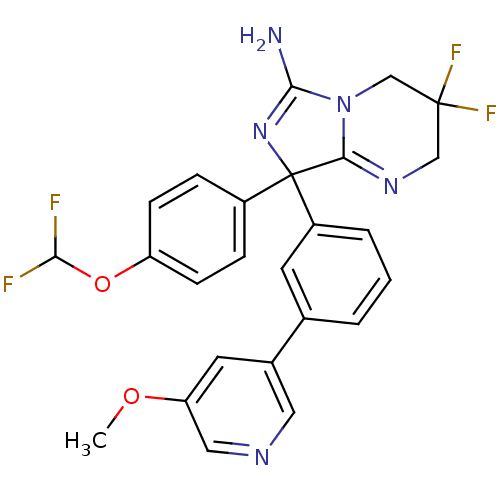 (CHEMBL1957475) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419863 (CHEMBL1957475) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419859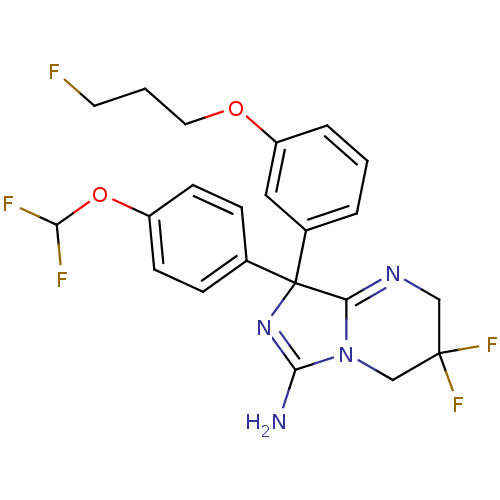 (CHEMBL1957482) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419862 (CHEMBL1957474) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419860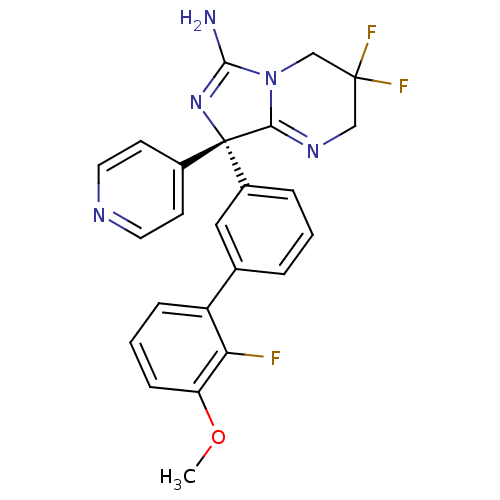 (CHEMBL1957471) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419872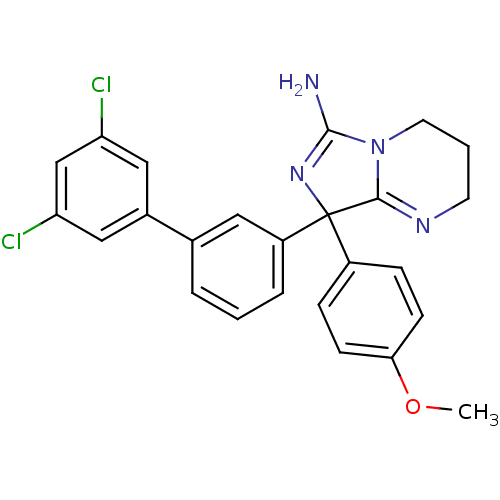 (CHEMBL1957469) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419862 (CHEMBL1957474) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419869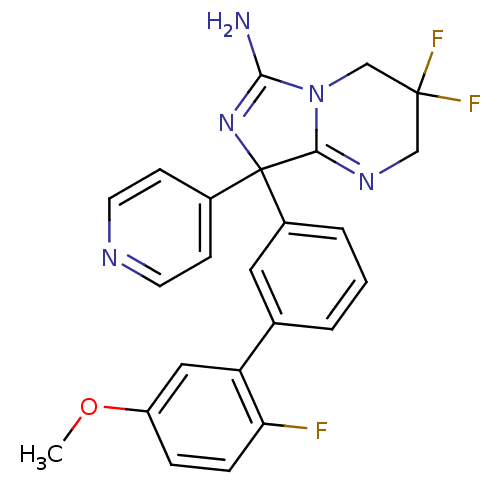 (CHEMBL1957477) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419864 (CHEMBL1957476) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419860 (CHEMBL1957471) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419871 (CHEMBL1957483) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419857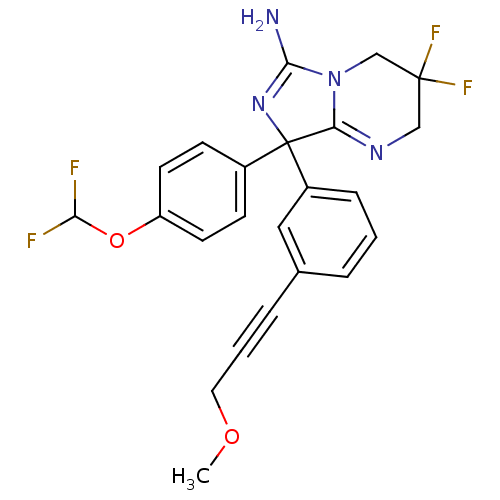 (CHEMBL1957480) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419865 (CHEMBL1957478) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419860 (CHEMBL1957471) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419865 (CHEMBL1957478) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419860 (CHEMBL1957471) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419858 (CHEMBL1957481) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419859 (CHEMBL1957482) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419857 (CHEMBL1957480) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419866 (CHEMBL1957468) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50300721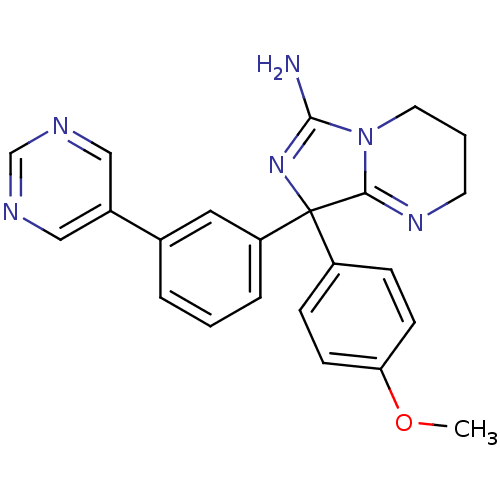 (8-(4-methoxyphenyl)-8-(3-(pyrimidin-5-yl)phenyl)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419861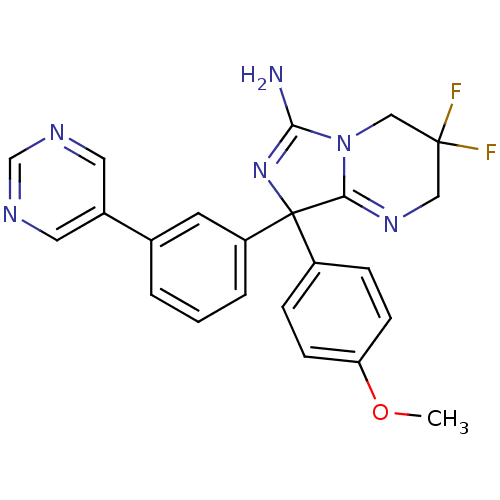 (CHEMBL1957473) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419868 (CHEMBL1957472) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419861 (CHEMBL1957473) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419867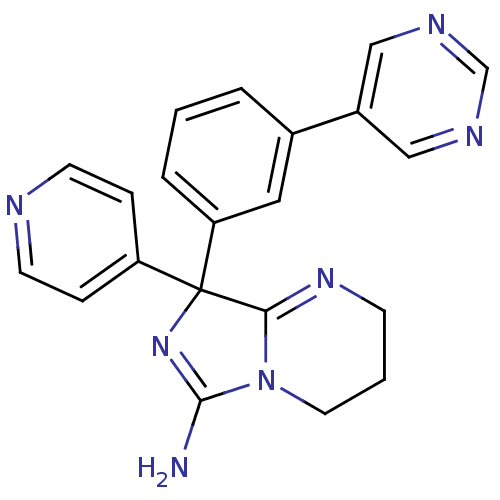 (CHEMBL1955882) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419869 (CHEMBL1957477) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419868 (CHEMBL1957472) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE-1-mediated sAPPbeta production in human SH-SY5Y cells after 16 hrs | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50419870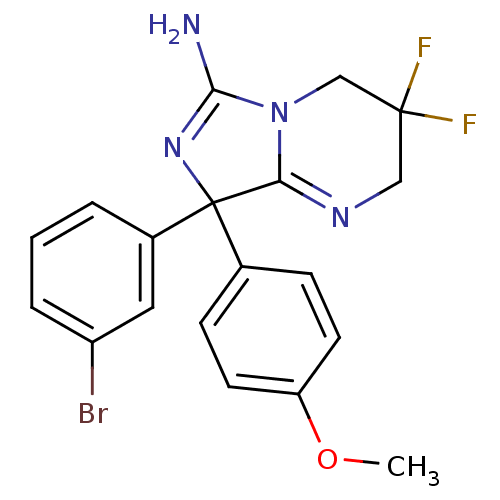 (CHEMBL1957479) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE-1 using (Eu)CEVNLDAEFK(Qsy7) as substrate preincubated for 10 mins prior substrate addition measured after 15 mins by FRET-b... | Bioorg Med Chem Lett 22: 1854-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.079 BindingDB Entry DOI: 10.7270/Q2Z60Q93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50118213 (5'-UTP | CHEMBL336296 | H4utp | UTP | uridine 5'-(...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y4 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50118213 (5'-UTP | CHEMBL336296 | H4utp | UTP | uridine 5'-(...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as intracellular calcium mobilization | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50205408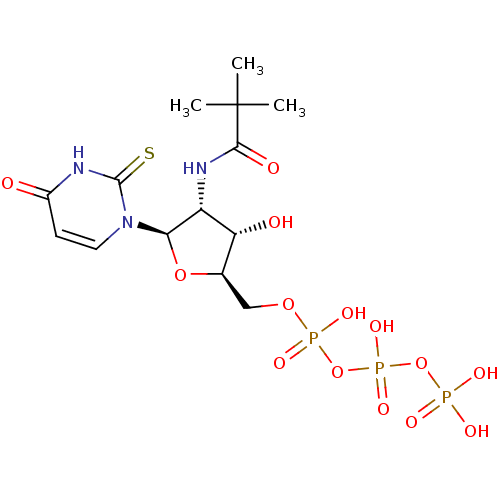 (2'-trifluoroacetylamino-2'-deoxy-2-thiouridine 5'-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199176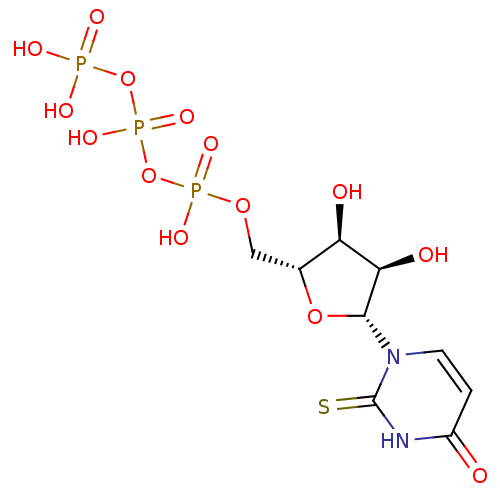 (2-thio-1-beta-D-ribofuranosyl(3H)pyrimidine-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50205407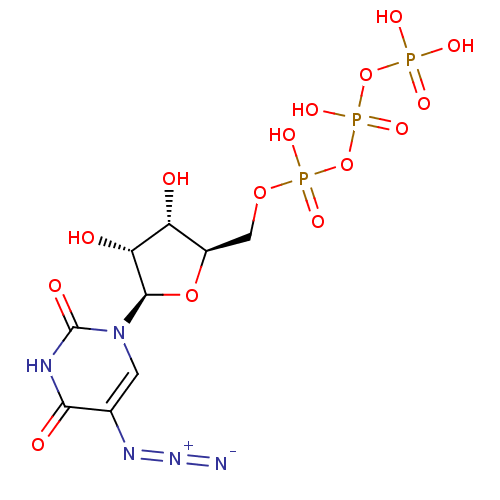 (({[({[(2R,3S,4R,5R)-5-(5-azido-2,4-dioxo-1,2,3,4-t...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50205406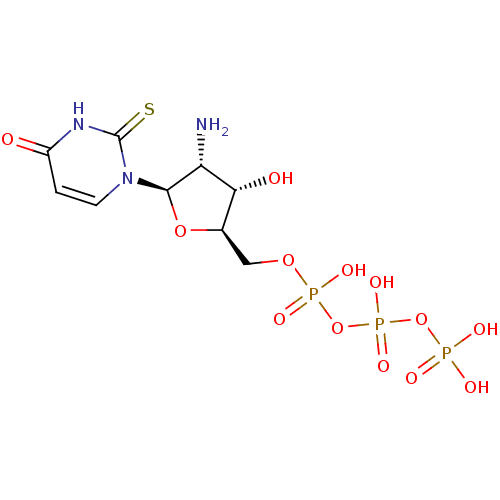 (((2R,3S,4R,5R)-4-amino-3-hydroxy-5-(4-oxo-2-thioxo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y4 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50205418 (CHEMBL410594 | uridine 5'-tetraphosphate 5'-ribose) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50199192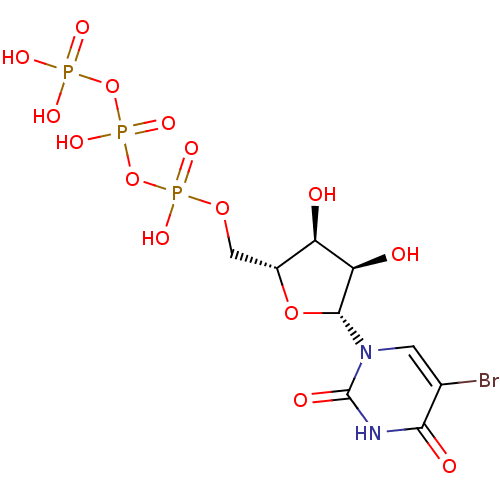 (((2R,3S,4R,5R)-5-(5-bromo-2,4-dioxo-3,4-dihydropyr...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50205417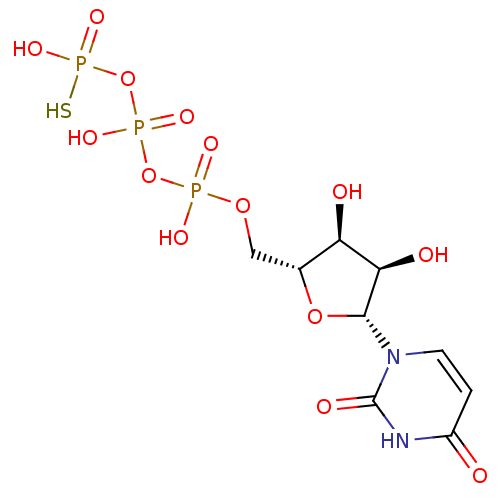 (CHEMBL220200 | UTP-gamma-S) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50205409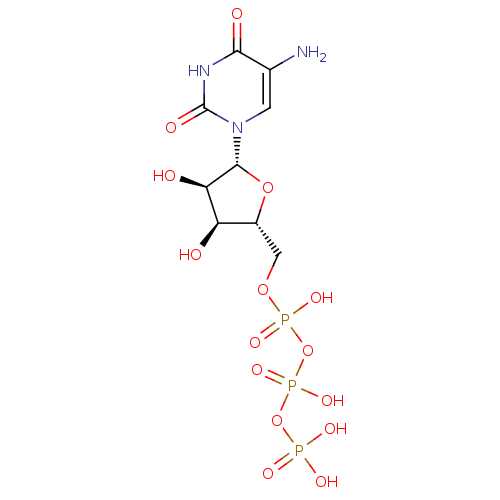 (({[({[(2R,3S,4R,5R)-5-(5-amino-2,4-dioxo-1,2,3,4-t...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 333 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50205415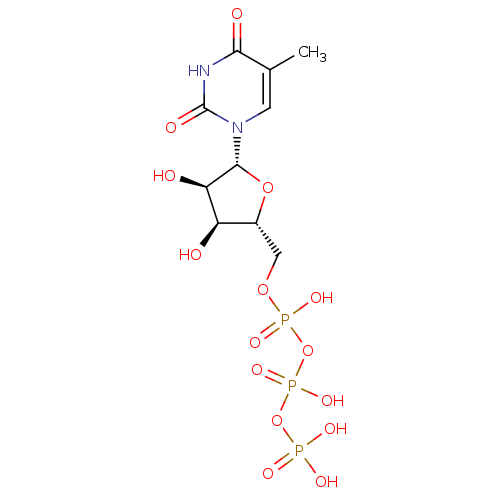 (({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(5-methyl-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y4 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50205416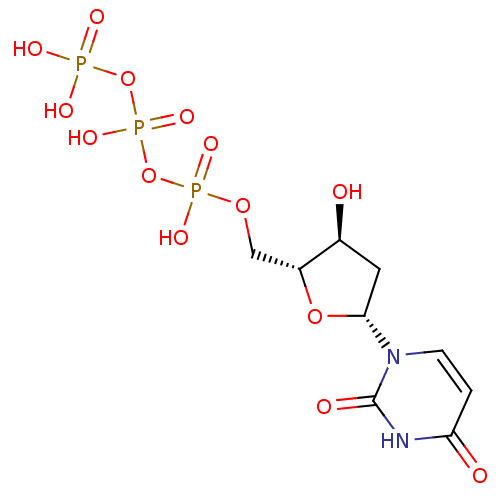 (2'-deoxyuridine 5'-(tetrahydrogen triphosphate) | ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50205415 (({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(5-methyl-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50205406 (((2R,3S,4R,5R)-4-amino-3-hydroxy-5-(4-oxo-2-thioxo...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50205413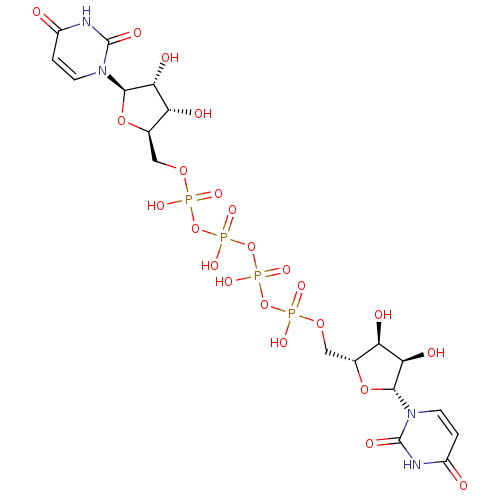 (CHEMBL221326 | P(1),P(4)-bis(uridin-5'-yl) tetraph...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as intracellular calcium mobilization | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50205405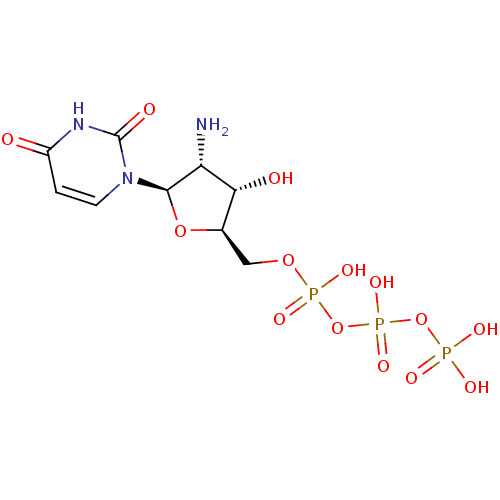 (({[({[(2R,3S,4R,5R)-4-amino-5-(2,4-dioxo-1,2,3,4-t...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50205414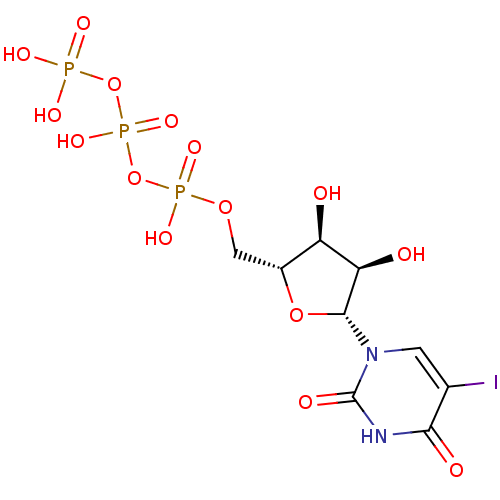 (({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(5-iodo-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 830 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50205413 (CHEMBL221326 | P(1),P(4)-bis(uridin-5'-yl) tetraph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50205415 (({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(5-methyl-2,4-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as stimulation of phospholipase C | J Med Chem 50: 1166-76 (2007) Article DOI: 10.1021/jm060903o BindingDB Entry DOI: 10.7270/Q2PK0GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |