

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130725 (3-(3-Chloro-4-hydroxy-phenylamino)-4-(2-nitro-phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using GSM as substrate incubated for 30 mins by luminescence based assay | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501616 (CHEMBL4084089) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501615 (CHEMBL4091846) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50535403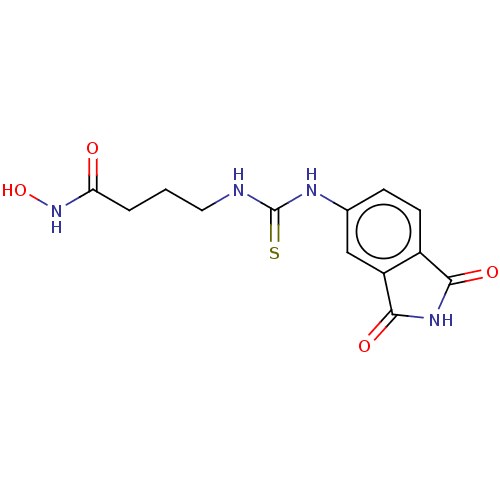 (CHEMBL4590543) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant his tagged HDAC1 expressed in baculovirus infected Sf6 insect cells using tertbutyloxycarbonyl (Boc)-(Ac)-Lys-7-amino... | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50535402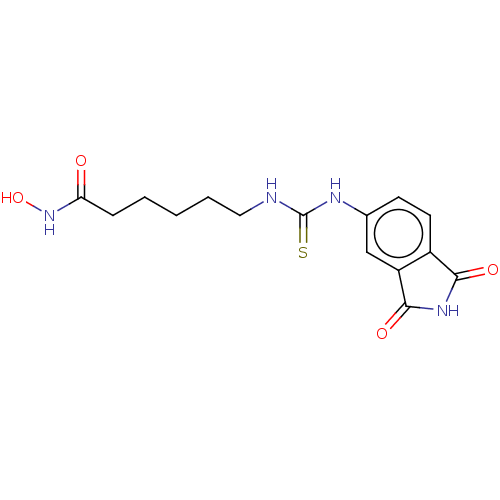 (CHEMBL4449010) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using GSM as substrate incubated for 30 mins by luminescence based assay | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50535402 (CHEMBL4449010) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant his tagged HDAC6 expressed in baculovirus infected Sf6 insect cells using batcp as substrate incubated for 1 hr by fl... | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50535401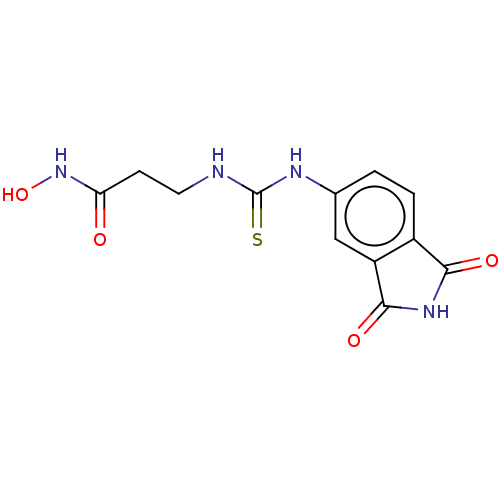 (CHEMBL4443876) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant his tagged HDAC1 expressed in baculovirus infected Sf6 insect cells using tertbutyloxycarbonyl (Boc)-(Ac)-Lys-7-amino... | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50535400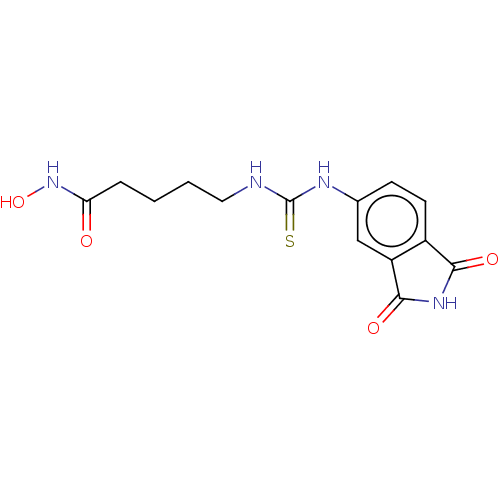 (CHEMBL4575045) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using GSM as substrate incubated for 30 mins by luminescence based assay | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50535400 (CHEMBL4575045) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant his tagged HDAC1 expressed in baculovirus infected Sf6 insect cells using tertbutyloxycarbonyl (Boc)-(Ac)-Lys-7-amino... | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501619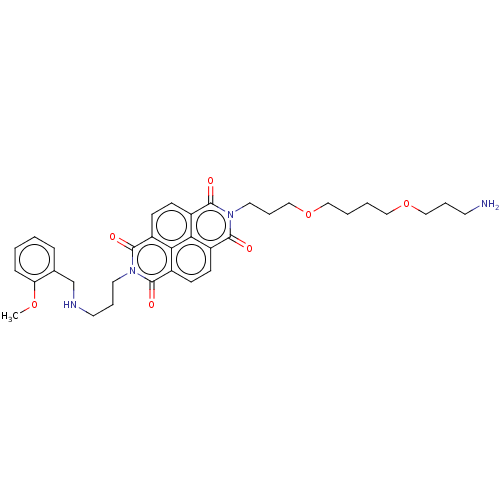 (CHEMBL4084827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501614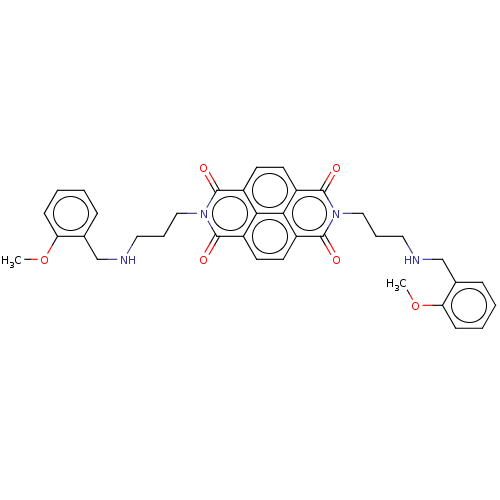 (CHEMBL583073) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501618 (CHEMBL4064888) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501617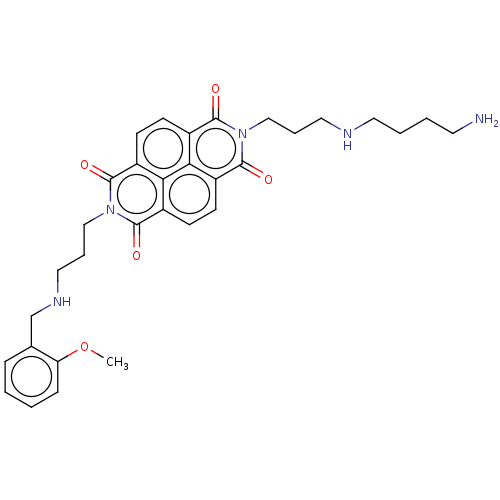 (CHEMBL4092588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50535403 (CHEMBL4590543) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using GSM as substrate incubated for 30 mins by luminescence based assay | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50535403 (CHEMBL4590543) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant his tagged HDAC6 expressed in baculovirus infected Sf6 insect cells using batcp as substrate incubated for 1 hr by fl... | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50535402 (CHEMBL4449010) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant his tagged HDAC1 expressed in baculovirus infected Sf6 insect cells using tertbutyloxycarbonyl (Boc)-(Ac)-Lys-7-amino... | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50535400 (CHEMBL4575045) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant his tagged HDAC6 expressed in baculovirus infected Sf6 insect cells using batcp as substrate incubated for 1 hr by fl... | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50535401 (CHEMBL4443876) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant his tagged HDAC6 expressed in baculovirus infected Sf6 insect cells using batcp as substrate incubated for 1 hr by fl... | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50535401 (CHEMBL4443876) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using GSM as substrate incubated for 30 mins by luminescence based assay | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50350644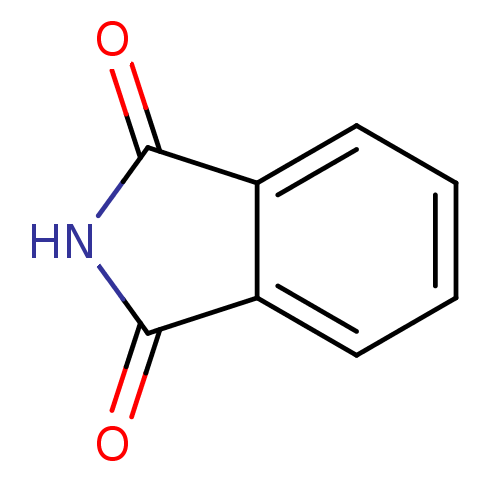 (PHTHALIMIDE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using GSM as substrate incubated for 30 mins by luminescence based assay | ACS Med Chem Lett 10: 469-474 (2019) Article DOI: 10.1021/acsmedchemlett.8b00507 BindingDB Entry DOI: 10.7270/Q2HD805P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50555892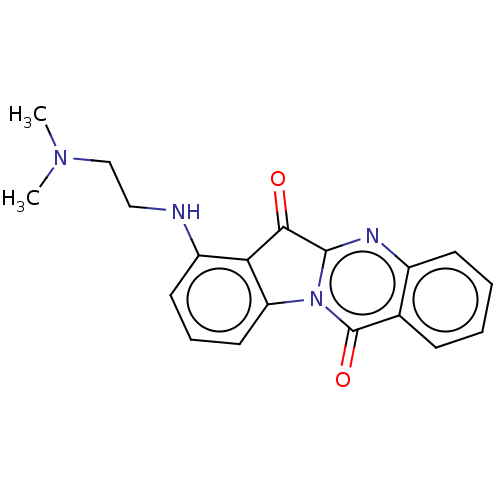 (CHEMBL4745205) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human DNA topoisomerase 2alpha assessed as suppression of decatenation using catenated kinetoplast DNA as substrate measured after 30 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112504 BindingDB Entry DOI: 10.7270/Q2VT1WRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50127140 ((-)-etoposide | (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human DNA topoisomerase 2alpha assessed as suppression of decatenation using catenated kinetoplast DNA as substrate measured after 30 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112504 BindingDB Entry DOI: 10.7270/Q2VT1WRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50555891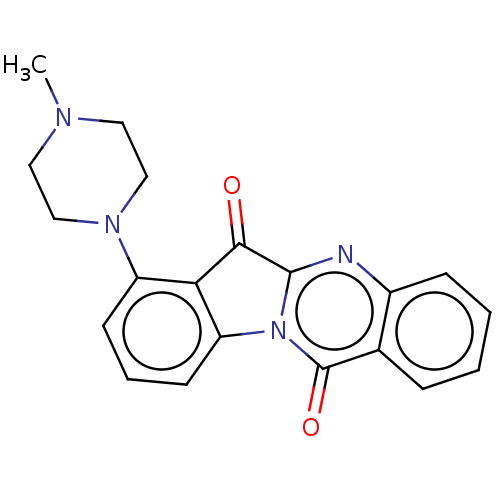 (CHEMBL4756707) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human DNA topoisomerase 2alpha assessed as suppression of decatenation using catenated kinetoplast DNA as substrate measured after 30 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112504 BindingDB Entry DOI: 10.7270/Q2VT1WRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50555893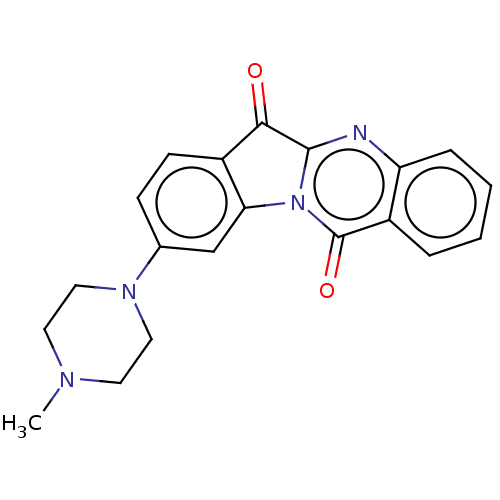 (CHEMBL4747945) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human DNA topoisomerase 2alpha assessed as suppression of decatenation using catenated kinetoplast DNA as substrate measured after 30 m... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112504 BindingDB Entry DOI: 10.7270/Q2VT1WRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||