 Found 1682 hits with Last Name = 'bharathan' and Initial = 'i'
Found 1682 hits with Last Name = 'bharathan' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023
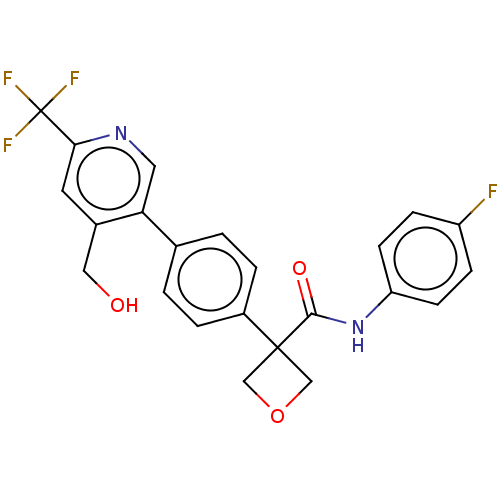
(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(MOUSE) | BDBM60536
(BDBM50399283 | US9102622, 13.2)Show InChI InChI=1S/C20H25FO3S/c21-25(22,23)15-8-3-1-2-5-10-18-13-9-14-20(16-18)24-17-19-11-6-4-7-12-19/h4,6-7,9,11-14,16H,1-3,5,8,10,15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells |
J Med Chem 55: 10074-89 (2012)
Article DOI: 10.1021/jm301205j
BindingDB Entry DOI: 10.7270/Q2J967HK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50399279

(CHEMBL2180822)Show InChI InChI=1S/C17H19FO4S/c18-23(19,20)13-5-4-12-21-16-8-10-17(11-9-16)22-14-15-6-2-1-3-7-15/h1-3,6-11H,4-5,12-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from CB1 receptor in rat brain homogenates |
J Med Chem 55: 10074-89 (2012)
Article DOI: 10.1021/jm301205j
BindingDB Entry DOI: 10.7270/Q2J967HK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50399282

(CHEMBL2180817 | US9102622, 13.4)Show InChI InChI=1S/C18H21FO3S/c19-23(20,21)14-6-2-5-7-16-10-12-18(13-11-16)22-15-17-8-3-1-4-9-17/h1,3-4,8-13H,2,5-7,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from CB1 receptor in rat brain homogenates |
J Med Chem 55: 10074-89 (2012)
Article DOI: 10.1021/jm301205j
BindingDB Entry DOI: 10.7270/Q2J967HK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50399281
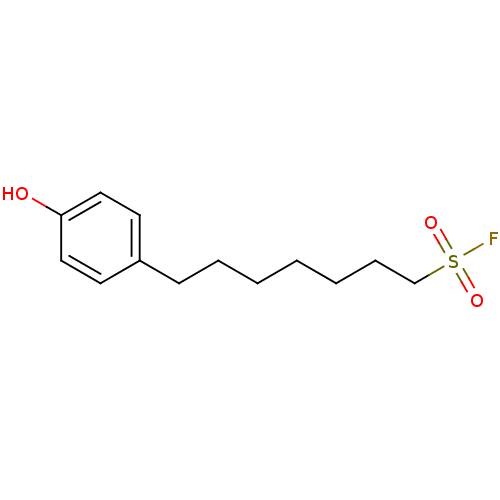
(CHEMBL2180818 | US9102622, 14.1)Show InChI InChI=1S/C13H19FO3S/c14-18(16,17)11-5-3-1-2-4-6-12-7-9-13(15)10-8-12/h7-10,15H,1-6,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from CB1 receptor in rat brain homogenates |
J Med Chem 55: 10074-89 (2012)
Article DOI: 10.1021/jm301205j
BindingDB Entry DOI: 10.7270/Q2J967HK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50399280

(CHEMBL2180821 | US9102622, 14.4)Show InChI InChI=1S/C11H15FO3S/c12-16(14,15)9-3-1-2-4-10-5-7-11(13)8-6-10/h5-8,13H,1-4,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from CB1 receptor in rat brain homogenates |
J Med Chem 55: 10074-89 (2012)
Article DOI: 10.1021/jm301205j
BindingDB Entry DOI: 10.7270/Q2J967HK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50399282

(CHEMBL2180817 | US9102622, 13.4)Show InChI InChI=1S/C18H21FO3S/c19-23(20,21)14-6-2-5-7-16-10-12-18(13-11-16)22-15-17-8-3-1-4-9-17/h1,3-4,8-13H,2,5-7,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells |
J Med Chem 55: 10074-89 (2012)
Article DOI: 10.1021/jm301205j
BindingDB Entry DOI: 10.7270/Q2J967HK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50399279

(CHEMBL2180822)Show InChI InChI=1S/C17H19FO4S/c18-23(19,20)13-5-4-12-21-16-8-10-17(11-9-16)22-14-15-6-2-1-3-7-15/h1-3,6-11H,4-5,12-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells |
J Med Chem 55: 10074-89 (2012)
Article DOI: 10.1021/jm301205j
BindingDB Entry DOI: 10.7270/Q2J967HK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM60536

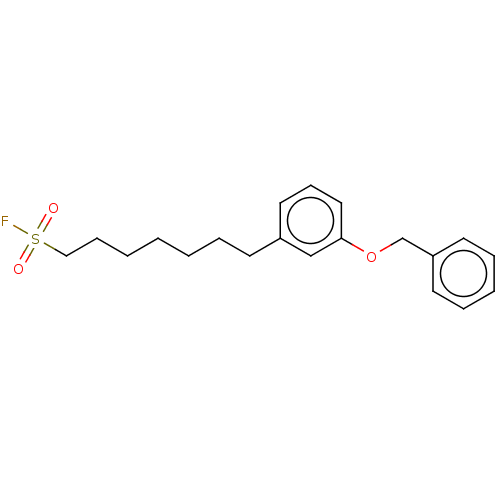
(BDBM50399283 | US9102622, 13.2)Show InChI InChI=1S/C20H25FO3S/c21-25(22,23)15-8-3-1-2-5-10-18-13-9-14-20(16-18)24-17-19-11-6-4-7-12-19/h4,6-7,9,11-14,16H,1-3,5,8,10,15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 335 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from CB1 receptor in rat brain homogenates |
J Med Chem 55: 10074-89 (2012)
Article DOI: 10.1021/jm301205j
BindingDB Entry DOI: 10.7270/Q2J967HK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50399280

(CHEMBL2180821 | US9102622, 14.4)Show InChI InChI=1S/C11H15FO3S/c12-16(14,15)9-3-1-2-4-10-5-7-11(13)8-6-10/h5-8,13H,1-4,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 577 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells |
J Med Chem 55: 10074-89 (2012)
Article DOI: 10.1021/jm301205j
BindingDB Entry DOI: 10.7270/Q2J967HK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50399281

(CHEMBL2180818 | US9102622, 14.1)Show InChI InChI=1S/C13H19FO3S/c14-18(16,17)11-5-3-1-2-4-6-12-7-9-13(15)10-8-12/h7-10,15H,1-6,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 698 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells |
J Med Chem 55: 10074-89 (2012)
Article DOI: 10.1021/jm301205j
BindingDB Entry DOI: 10.7270/Q2J967HK |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604021
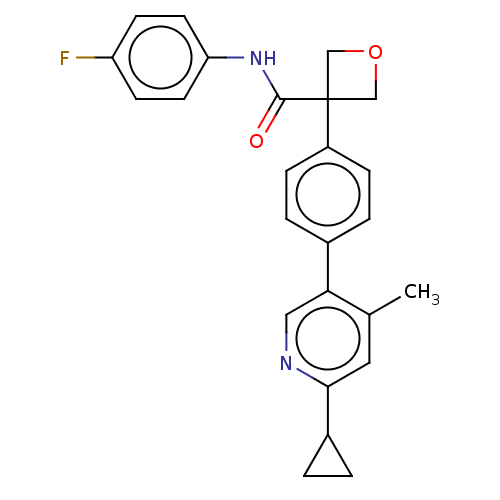
(CHEMBL5207194)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503
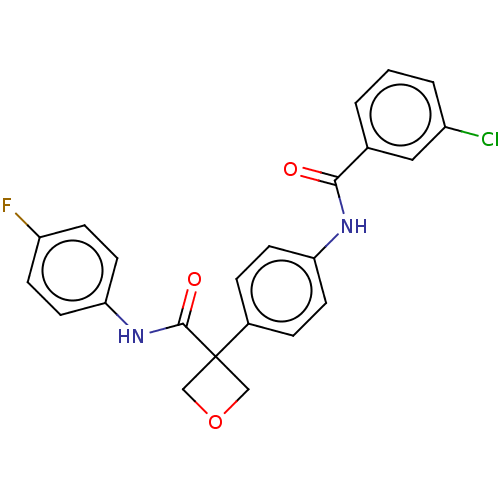
(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM50572114
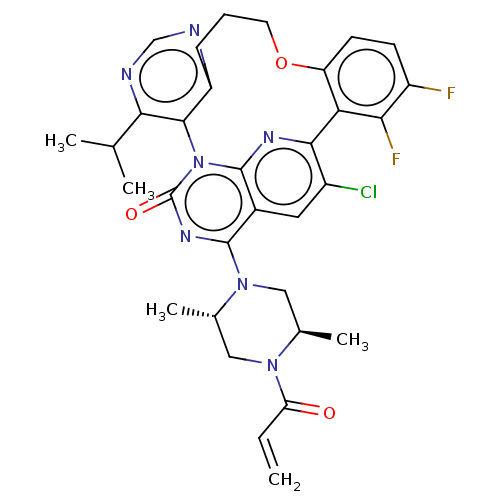
(CHEMBL4852458 | US20240043448, Example 74)Show SMILES CC(C)c1ncnc2CCCOc3ccc(F)c(F)c3-c3nc4n(-c12)c(=O)nc(N1C[C@@H](C)N(C[C@@H]1C)C(=O)C=C)c4cc3Cl |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM650996
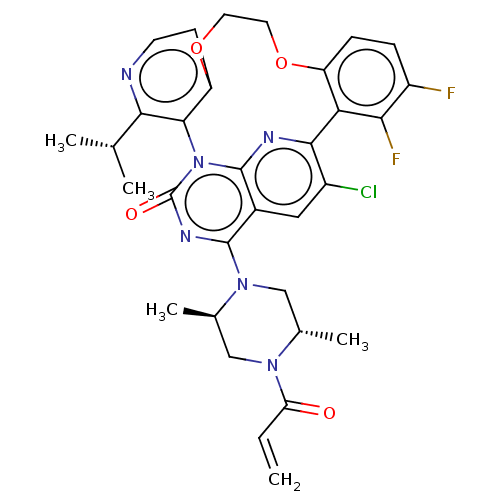
((5aSa,17aRa)-20-chloro-2- [(2S,5R)-2,5-dimethyl-4-...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM651033
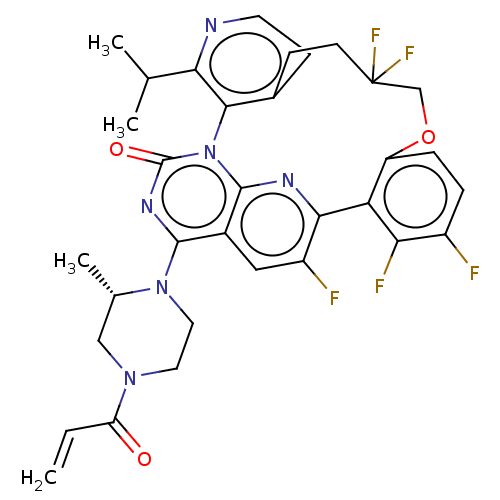
(US20240043448, Example 97) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM651028

(2'-[(2S,5R)-2,5-dimethyl-4-(prop-2- enoyl)piperazi...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM650997
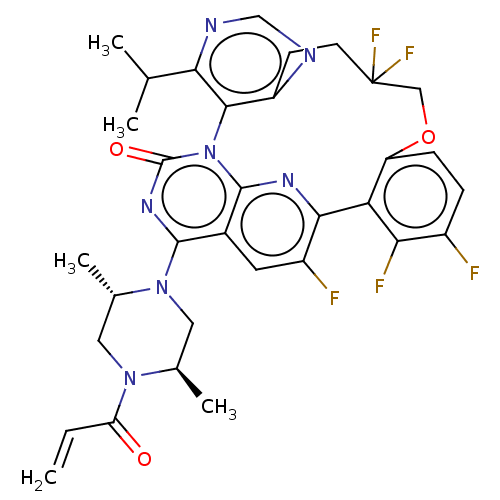
(2-[(2S,5R)-2,5-dimethyl-4-(prop- 2-enoyl)piperazin...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552941

(CHEMBL4764710)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OC1CCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604025
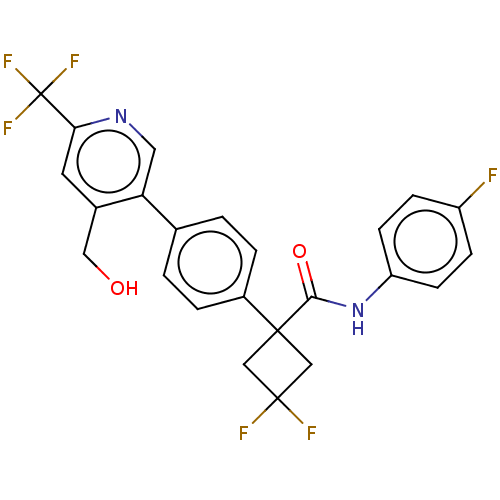
(CHEMBL5173861)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CC(F)(F)C1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM50572113
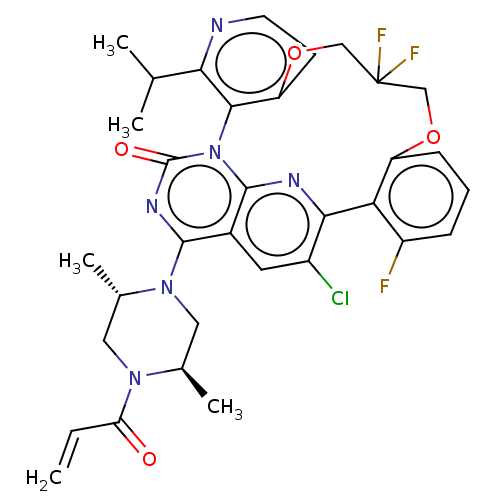
(CHEMBL4879271 | US20240043448, Example 59)Show SMILES CC(C)c1nccc2OCC(F)(F)COc3cccc(F)c3-c3nc4n(-c12)c(=O)nc(N1C[C@@H](C)N(C[C@@H]1C)C(=O)C=C)c4cc3Cl |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50362985

(CHEMBL1945502)Show SMILES COc1ccc(Nc2ncc3CC(=S)Nc4cc(Cl)ccc4-c3n2)cc1CCCN(C)C Show InChI InChI=1S/C24H26ClN5OS/c1-30(2)10-4-5-15-11-18(7-9-21(15)31-3)27-24-26-14-16-12-22(32)28-20-13-17(25)6-8-19(20)23(16)29-24/h6-9,11,13-14H,4-5,10,12H2,1-3H3,(H,28,32)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 55: 197-208 (2012)
Article DOI: 10.1021/jm2011172
BindingDB Entry DOI: 10.7270/Q21C1XBR |
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM650991
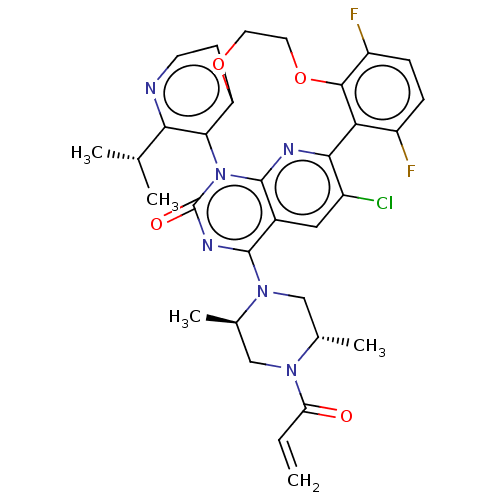
(US20240043448, Example 51-1) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM650995
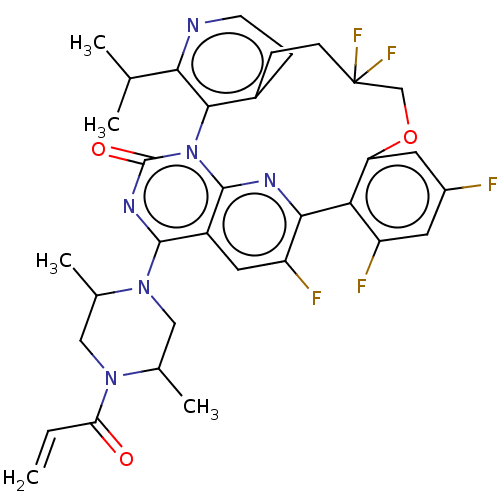
((5aRa,18aRa)-2-[(2S,5R)-2,5- dimethyl-4-(prop-2- e...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM651024
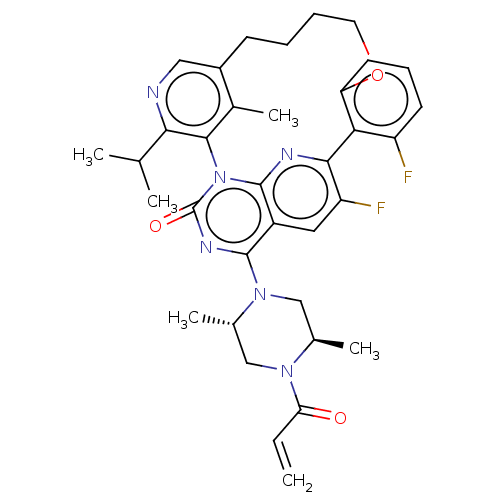
(2-[(2S,5R)-2,5-dimethyl-4-(prop-2- enoyl)piperazin...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM650990

(US20240043448, Example 50) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50573261

(CHEMBL4862297)Show SMILES C[C@H]1C[C@@]1(NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(C)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00265
BindingDB Entry DOI: 10.7270/Q20K2DBP |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50573260
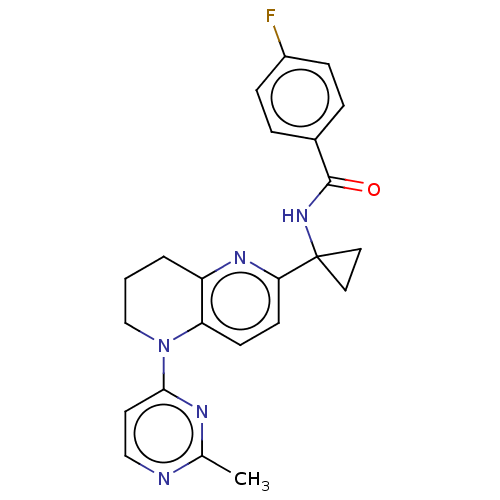
(CHEMBL4859249)Show SMILES Cc1nccc(n1)N1CCCc2nc(ccc12)C1(CC1)NC(=O)c1ccc(F)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00265
BindingDB Entry DOI: 10.7270/Q20K2DBP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50573259
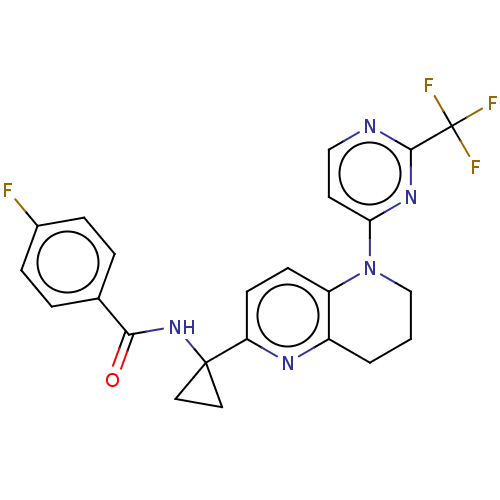
(CHEMBL4847724)Show SMILES Fc1ccc(cc1)C(=O)NC1(CC1)c1ccc2N(CCCc2n1)c1ccnc(n1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00265
BindingDB Entry DOI: 10.7270/Q20K2DBP |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604024

(CHEMBL5193283)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CCC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM50572117

(CHEMBL4863593 | US20240043448, Example 91)Show SMILES CC(C)c1nccc2C(C)CCOc3ccc(F)c(F)c3-c3nc4n(-c12)c(=O)nc(N1C[C@@H](C)N(C[C@@H]1C)C(=O)C=C)c4cc3F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552934

(CHEMBL4744727)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM650999
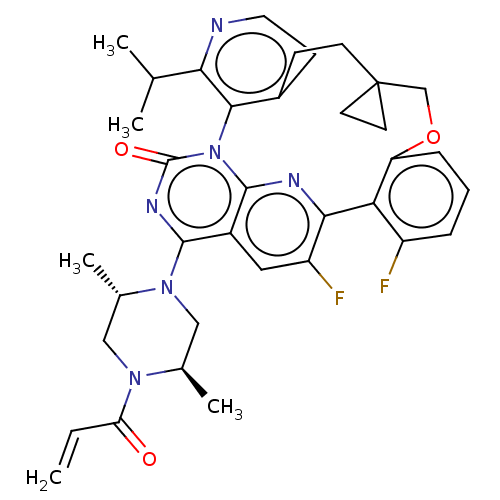
(2'-[(2S,5R)-2,5-dimethyl-4-(prop- 2-enoyl)piperazi...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604025

(CHEMBL5173861)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CC(F)(F)C1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552931

(CHEMBL4755227)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCCc2n1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552948

(CHEMBL4779920)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccncn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM50572115

(CHEMBL4864188 | US20240043448, Example 75)Show SMILES CC(C)c1nccc2CCCOc3cccc(F)c3-c3nc4n(-c12)c(=O)nc(N1C[C@@H](C)N(C[C@@H]1C)C(=O)C=C)c4cc3F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM651008
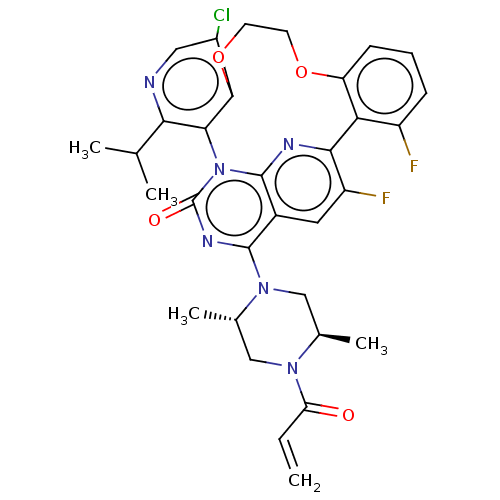
(US20240043448, Example 70) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552950

(CHEMBL4783395)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00265
BindingDB Entry DOI: 10.7270/Q20K2DBP |
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM650992
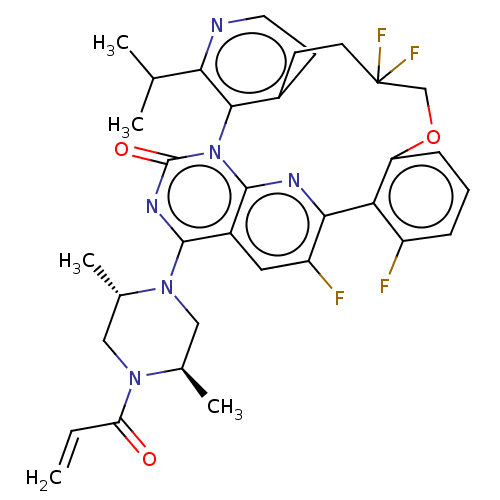
(US20240043448, Example 52) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552950

(CHEMBL4783395)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023

(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50573262

(CHEMBL4870929)Show SMILES [H][C@@]12C[C@]1([H])c1nc(ccc1N(C2)c1ccnc(C)n1)C1(CC1)NC(=O)c1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00265
BindingDB Entry DOI: 10.7270/Q20K2DBP |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503

(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604022
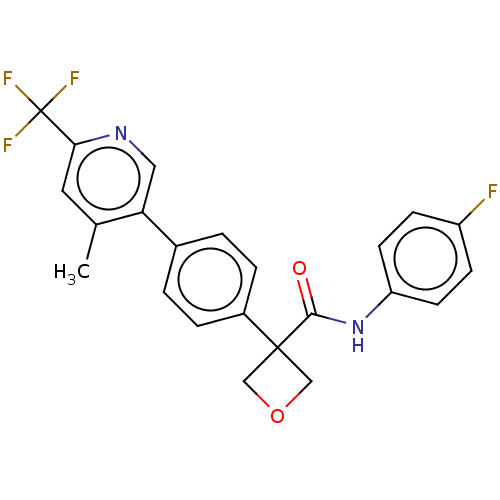
(CHEMBL5180607)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604021

(CHEMBL5207194)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM651045
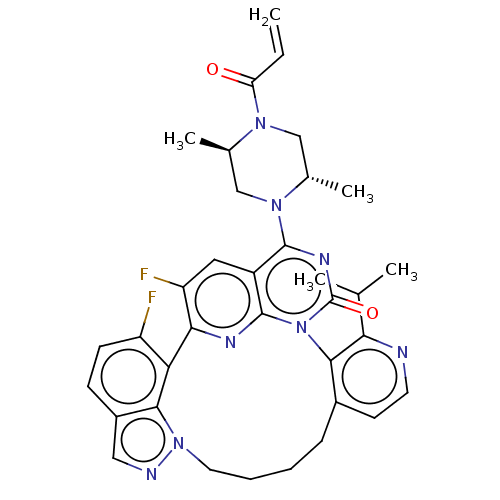
(US20240043448, Example 109) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
GTPase KRas [2-169,G12C,C51S,C80L,C118S]
(Human sapiens (Human)) | BDBM50572116

(CHEMBL4855378 | US20240043448, Example 76)Show SMILES CC(C)c1nccc2CCCOc3cccc(Cl)c3-c3nc4n(-c12)c(=O)nc(N1C[C@@H](C)N(C[C@@H]1C)C(=O)C=C)c4cc3F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data