

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM17290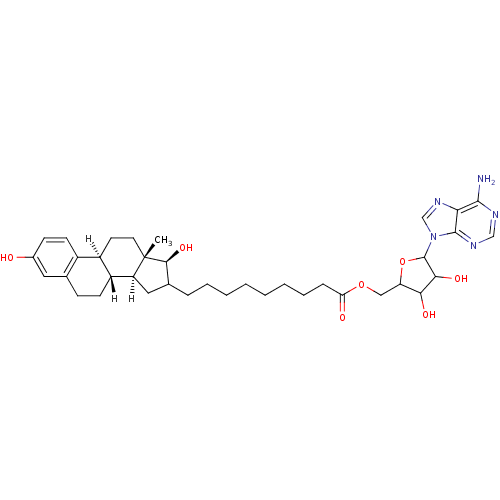 (E2-adenosine hybrid compound, 8 | EM-1745 | EM1745...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CHUL | Assay Description For steady-state kinetic study of hybrid inhibitors, a Fluorolog 3 instrument was used to monitor the fluorescent signal of NADPH formed during estra... | FASEB J 16: 1829-31 (2002) Article DOI: 10.1096/fj.02-0026fje BindingDB Entry DOI: 10.7270/Q23T9FGW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM17293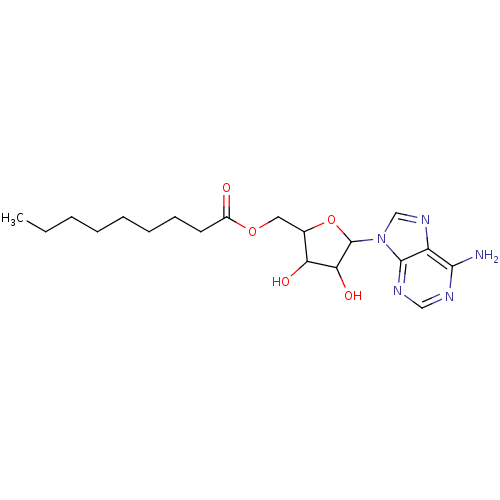 (Compound 10 | MB-329-131A2 | [5-(6-amino-9H-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.50E+5 | -21.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
CHUL | Assay Description For steady-state kinetic study of hybrid inhibitors, a Fluorolog 3 instrument was used to monitor the fluorescent signal of NADPH formed during estra... | FASEB J 16: 1829-31 (2002) Article DOI: 10.1096/fj.02-0026fje BindingDB Entry DOI: 10.7270/Q23T9FGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369432 (CHEMBL1627465) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Research Center Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit human embryonal kidney cell derived steroid sulfatase activity in transforming [3H]E1S (estrone sulfat... | J Med Chem 42: 2280-6 (1999) Article DOI: 10.1021/jm980677l BindingDB Entry DOI: 10.7270/Q2SN09M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369431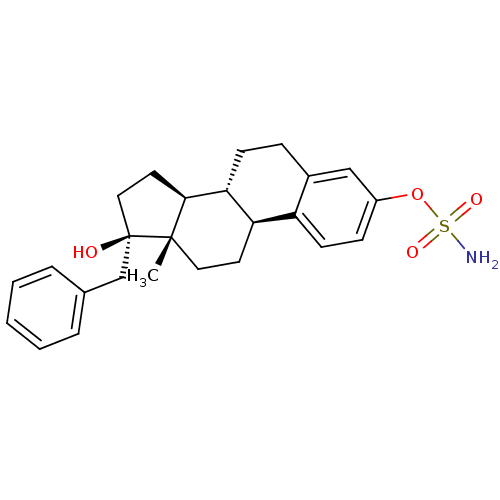 (CHEMBL1627878) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Research Center Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit human embryonal kidney cell derived steroid sulfatase activity in transforming [3H]E1S (estrone sulfat... | J Med Chem 42: 2280-6 (1999) Article DOI: 10.1021/jm980677l BindingDB Entry DOI: 10.7270/Q2SN09M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369432 (CHEMBL1627465) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Research Center Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the transformation of [C14]-DHEAS to DHEA by steroid sulfatase derived from human embryonal kidney cel... | J Med Chem 42: 2280-6 (1999) Article DOI: 10.1021/jm980677l BindingDB Entry DOI: 10.7270/Q2SN09M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369740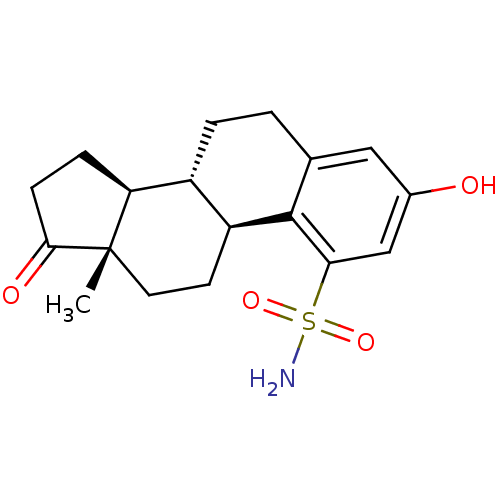 (CHEMBL1627637) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity by the compound was determined in human embryonic kidney (HEK)-293 cells transfected with a sulfatase expres... | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134329 (CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Research Center Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit human embryonal kidney cell derived steroid sulfatase activity in transforming [3H]E1S (estrone sulfat... | J Med Chem 42: 2280-6 (1999) Article DOI: 10.1021/jm980677l BindingDB Entry DOI: 10.7270/Q2SN09M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369740 (CHEMBL1627637) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity by the compound was determined in human embryonic kidney (HEK)-293 cells transfected with a sulfatase expres... | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369431 (CHEMBL1627878) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Research Center Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the transformation of [C14]-DHEAS to DHEA by steroid sulfatase derived from human embryonal kidney cel... | J Med Chem 42: 2280-6 (1999) Article DOI: 10.1021/jm980677l BindingDB Entry DOI: 10.7270/Q2SN09M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50134329 (CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Research Center Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the transformation of [C14]-DHEAS to DHEA by steroid sulfatase derived from human embryonal kidney cel... | J Med Chem 42: 2280-6 (1999) Article DOI: 10.1021/jm980677l BindingDB Entry DOI: 10.7270/Q2SN09M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366532 (CHEMBL518966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Research Center Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit human embryonal kidney cell derived steroid sulfatase activity in transforming [3H]E1S (estrone sulfat... | J Med Chem 42: 2280-6 (1999) Article DOI: 10.1021/jm980677l BindingDB Entry DOI: 10.7270/Q2SN09M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366532 (CHEMBL518966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Steroid sulfatase activity was determined in human embryonic kidney (HEK)-293 cells transfected with a sulfatase expression vector (pCMV-sulfa) using... | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366532 (CHEMBL518966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Steroid sulfatase activity was determined in human embryonic kidney (HEK)-293 cells transfected with a sulfatase expression vector (pCMV-sulfa) using... | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366532 (CHEMBL518966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Research Center Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the transformation of [C14]-DHEAS to DHEA by steroid sulfatase derived from human embryonal kidney cel... | J Med Chem 42: 2280-6 (1999) Article DOI: 10.1021/jm980677l BindingDB Entry DOI: 10.7270/Q2SN09M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369744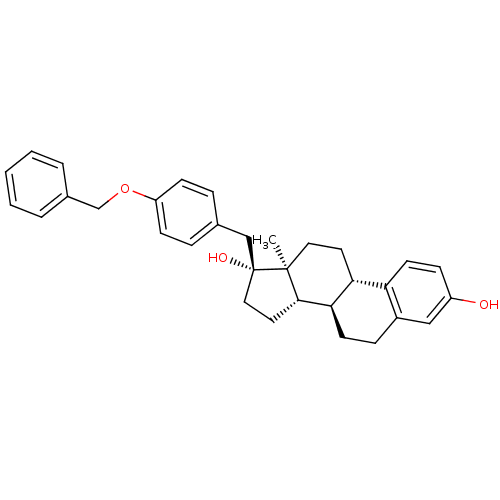 (CHEMBL1627617) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50363594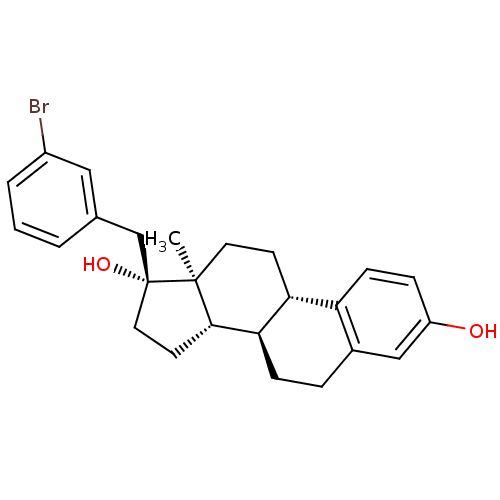 (CHEMBL1627429) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Curated by ChEMBL | Assay Description Estrone sulfatase activity against homogenized human JEG-3 cells was determined by measuring the [3H]E1 obtained from [3H]E1S | Bioorg Med Chem Lett 8: 1891-6 (1999) BindingDB Entry DOI: 10.7270/Q2PV6KWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50363594 (CHEMBL1627429) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369736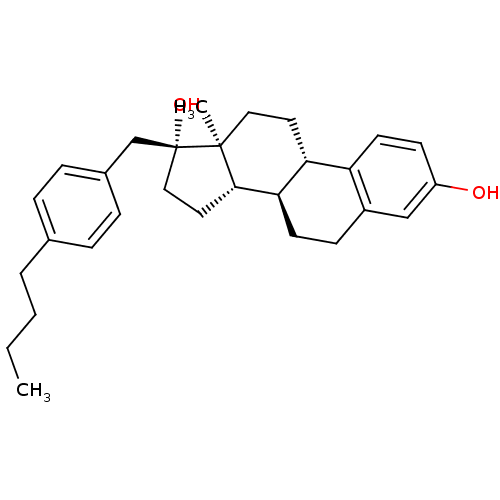 (CHEMBL1627626) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366532 (CHEMBL518966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366532 (CHEMBL518966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Curated by ChEMBL | Assay Description Estrone sulfatase activity against homogenized human JEG-3 cells was determined by measuring the [3H]E1 obtained from [3H]E1S | Bioorg Med Chem Lett 8: 1891-6 (1999) BindingDB Entry DOI: 10.7270/Q2PV6KWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM17290 (E2-adenosine hybrid compound, 8 | EM-1745 | EM1745...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.4 | 37 |
CHUL | Assay Description The enzymatic reaction was performed in the reaction buffer containing substrate, [14C]-estrone, and the test inhibitors. After the reaction, radiola... | FASEB J 16: 1829-31 (2002) Article DOI: 10.1096/fj.02-0026fje BindingDB Entry DOI: 10.7270/Q23T9FGW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50179201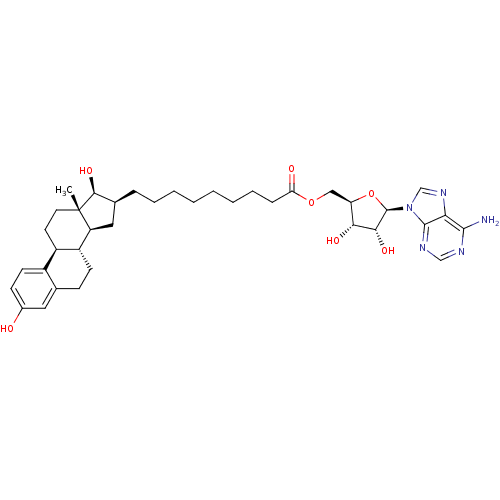 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ-Pavillon CHUL and Université Laval Curated by ChEMBL | Assay Description Inhibitory activity against type 1 17beta-HSD expressed in transfected HEK293 cells | J Med Chem 48: 8134-47 (2005) Article DOI: 10.1021/jm058235e BindingDB Entry DOI: 10.7270/Q20R9P0W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369739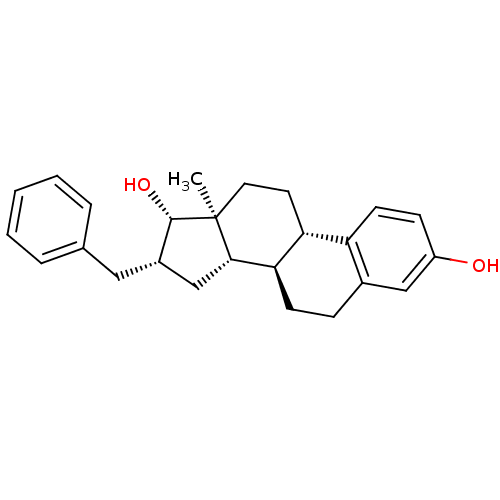 (CHEMBL270250) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells by the compound at 20 uM, activity was determined by considering total labeled estrone ([3H]E... | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366522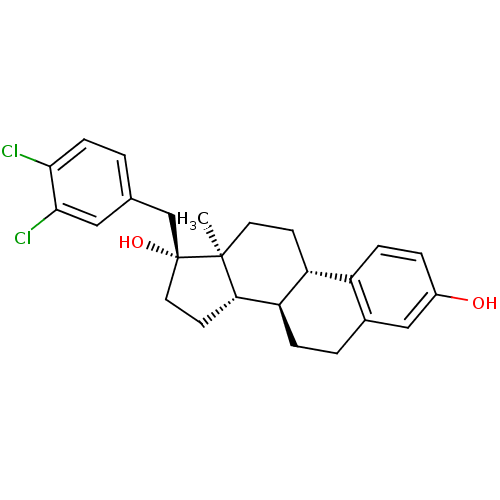 (CHEMBL1628091) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Curated by ChEMBL | Assay Description Estrone sulfatase activity against homogenized human JEG-3 cells was determined by measuring the [3H]E1 obtained from [3H]E1S | Bioorg Med Chem Lett 8: 1891-6 (1999) BindingDB Entry DOI: 10.7270/Q2PV6KWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366522 (CHEMBL1628091) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50179199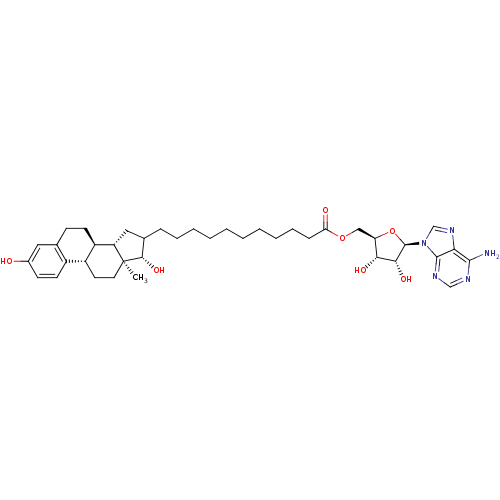 (5'-O-{11-[3',17'beta-dihydroxy-1',3',5'(10')-estra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ-Pavillon CHUL and Université Laval Curated by ChEMBL | Assay Description Inhibitory activity against type 1 17beta-HSD expressed in transfected HEK293 cells | J Med Chem 48: 8134-47 (2005) Article DOI: 10.1021/jm058235e BindingDB Entry DOI: 10.7270/Q20R9P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM17288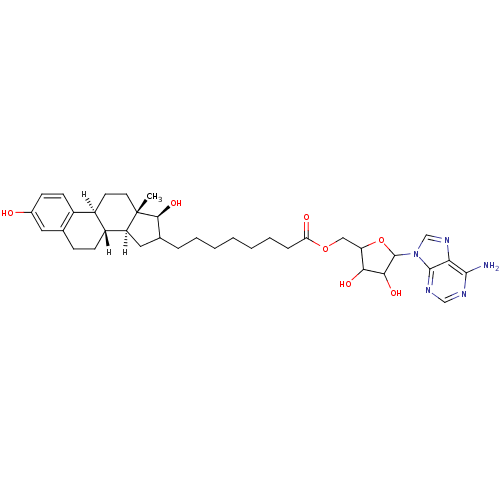 (E2-adenosine hybrid compound, 7 | [5-(6-amino-9H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | 7.4 | 37 |
CHUL | Assay Description The enzymatic reaction was performed in the reaction buffer containing substrate, [14C]-estrone, and the test inhibitors. After the reaction, radiola... | FASEB J 16: 1829-31 (2002) Article DOI: 10.1096/fj.02-0026fje BindingDB Entry DOI: 10.7270/Q23T9FGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50179198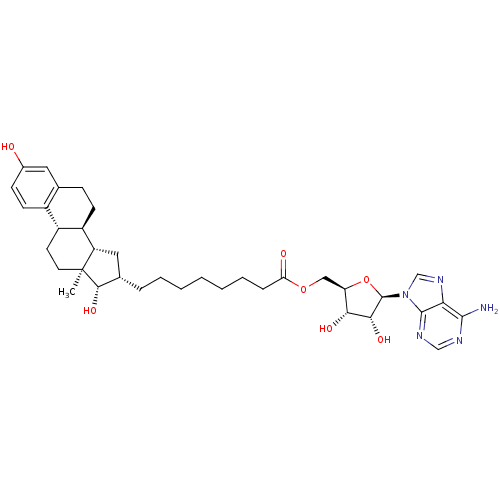 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ-Pavillon CHUL and Université Laval Curated by ChEMBL | Assay Description Inhibitory activity against type 1 17beta-HSD expressed in transfected HEK293 cells | J Med Chem 48: 8134-47 (2005) Article DOI: 10.1021/jm058235e BindingDB Entry DOI: 10.7270/Q20R9P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366529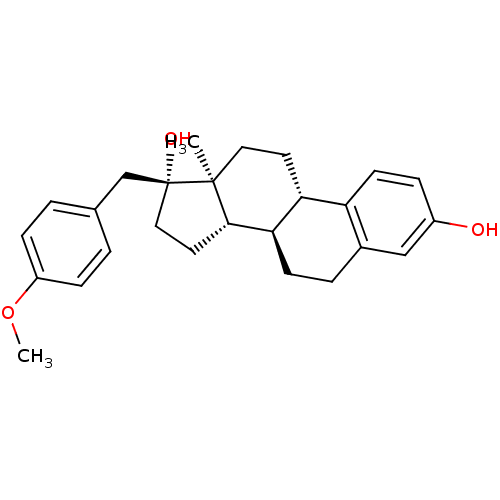 (CHEMBL1627421) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366525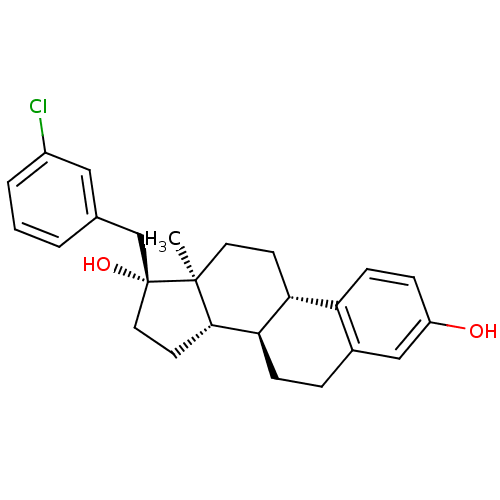 (CHEMBL1627418) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Curated by ChEMBL | Assay Description Estrone sulfatase activity against homogenized human JEG-3 cells was determined by measuring the [3H]E1 obtained from [3H]E1S | Bioorg Med Chem Lett 8: 1891-6 (1999) BindingDB Entry DOI: 10.7270/Q2PV6KWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366529 (CHEMBL1627421) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Curated by ChEMBL | Assay Description Estrone sulfatase activity against homogenized human JEG-3 cells was determined by measuring the [3H]E1 obtained from [3H]E1S | Bioorg Med Chem Lett 8: 1891-6 (1999) BindingDB Entry DOI: 10.7270/Q2PV6KWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366525 (CHEMBL1627418) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50179200 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ-Pavillon CHUL and Université Laval Curated by ChEMBL | Assay Description Inhibitory activity against type 1 17beta-HSD expressed in transfected HEK293 cells | J Med Chem 48: 8134-47 (2005) Article DOI: 10.1021/jm058235e BindingDB Entry DOI: 10.7270/Q20R9P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369746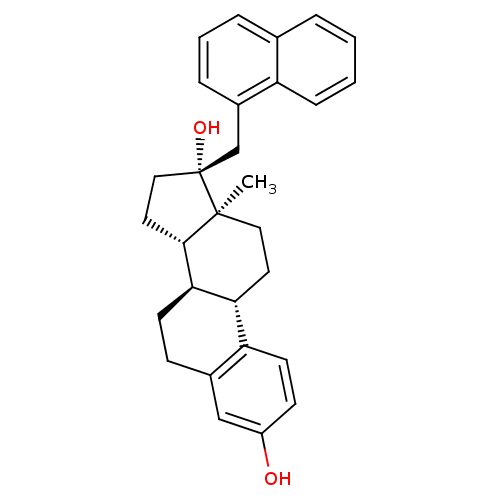 (CHEMBL1627953) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369733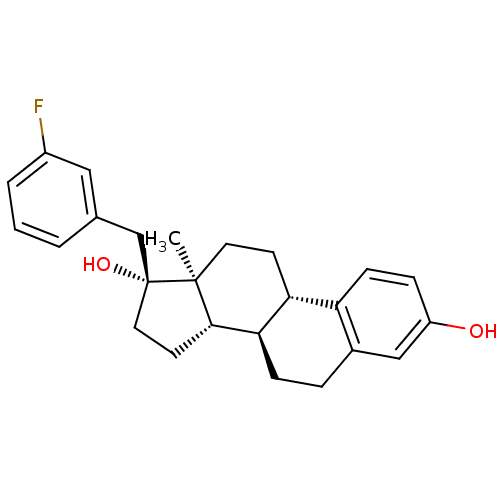 (CHEMBL1627640) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50179194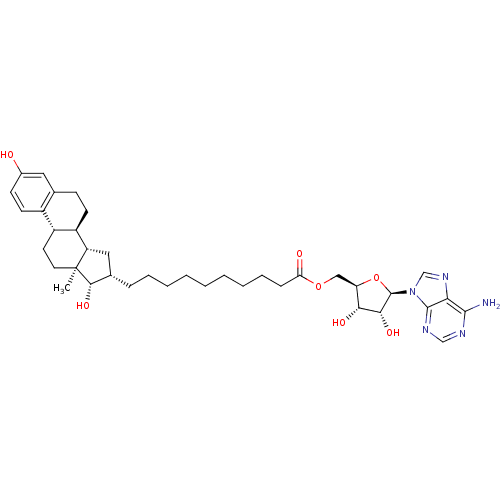 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ-Pavillon CHUL and Université Laval Curated by ChEMBL | Assay Description Inhibitory activity against type 1 17beta-HSD expressed in transfected HEK293 cells | J Med Chem 48: 8134-47 (2005) Article DOI: 10.1021/jm058235e BindingDB Entry DOI: 10.7270/Q20R9P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM17291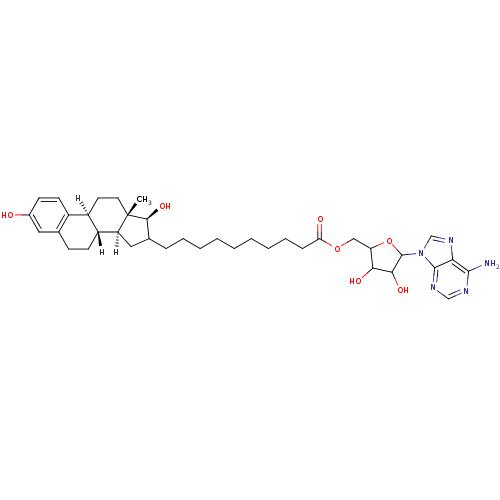 (E2-adenosine hybrid compound, 9 | [5-(6-amino-9H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.4 | 37 |
CHUL | Assay Description The enzymatic reaction was performed in the reaction buffer containing substrate, [14C]-estrone, and the test inhibitors. After the reaction, radiola... | FASEB J 16: 1829-31 (2002) Article DOI: 10.1096/fj.02-0026fje BindingDB Entry DOI: 10.7270/Q23T9FGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50355372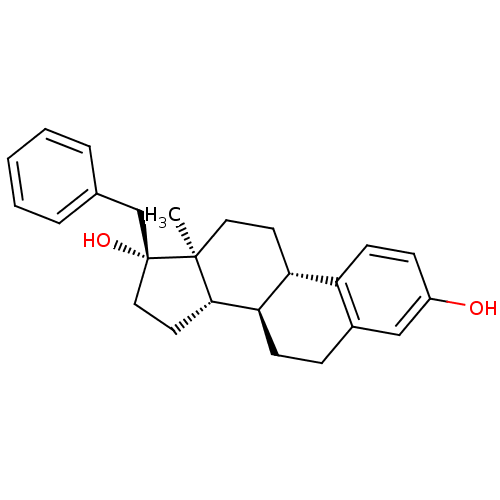 (CHEMBL482212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Steroid sulfatase activity was determined in human embryonic kidney (HEK)-293 cells transfected with a sulfatase expression vector (pCMV-sulfa) using... | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50355372 (CHEMBL482212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Research Center Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit human embryonal kidney cell derived steroid sulfatase activity in transforming [3H]E1S (estrone sulfat... | J Med Chem 42: 2280-6 (1999) Article DOI: 10.1021/jm980677l BindingDB Entry DOI: 10.7270/Q2SN09M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50355372 (CHEMBL482212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Curated by ChEMBL | Assay Description Estrone sulfatase activity against homogenized human JEG-3 cells was determined by measuring the [3H]E1 obtained from [3H]E1S | Bioorg Med Chem Lett 8: 1891-6 (1999) BindingDB Entry DOI: 10.7270/Q2PV6KWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50179195 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ-Pavillon CHUL and Université Laval Curated by ChEMBL | Assay Description Inhibitory activity against type 1 17beta-HSD expressed in transfected HEK293 cells | J Med Chem 48: 8134-47 (2005) Article DOI: 10.1021/jm058235e BindingDB Entry DOI: 10.7270/Q20R9P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50355372 (CHEMBL482212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50355372 (CHEMBL482212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Research Center Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit the transformation of [C14]-DHEAS to DHEA by steroid sulfatase derived from human embryonal kidney cel... | J Med Chem 42: 2280-6 (1999) Article DOI: 10.1021/jm980677l BindingDB Entry DOI: 10.7270/Q2SN09M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50355372 (CHEMBL482212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Steroid sulfatase activity was determined in human embryonic kidney (HEK)-293 cells transfected with a sulfatase expression vector (pCMV-sulfa) using... | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50366341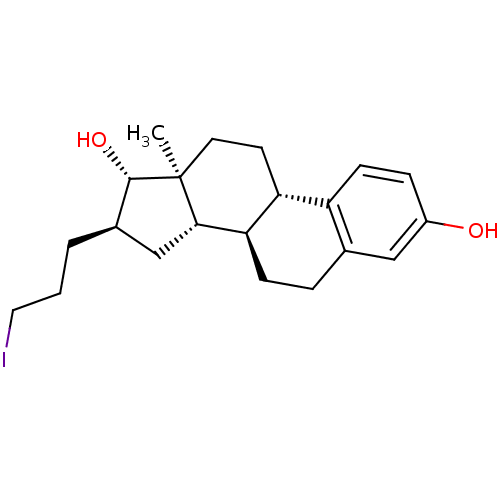 (CHEMBL1627453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of 17-beta-hydroxysteroid dehydrogenase type 1(17-beta-HSD type 1) | Bioorg Med Chem Lett 4: 2129-2132 (1994) Article DOI: 10.1016/S0960-894X(01)80115-3 BindingDB Entry DOI: 10.7270/Q2JW8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50179197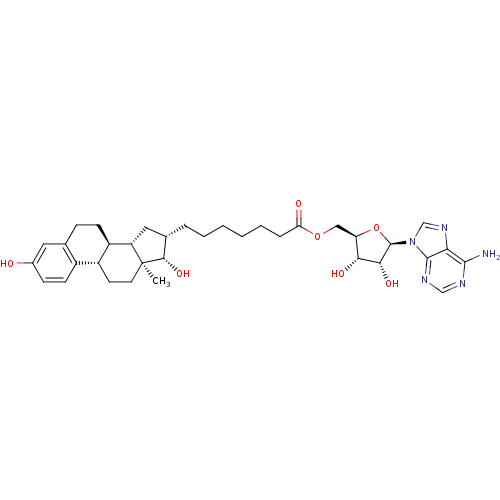 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ-Pavillon CHUL and Université Laval Curated by ChEMBL | Assay Description Inhibitory activity against type 1 17beta-HSD expressed in transfected HEK293 cells | J Med Chem 48: 8134-47 (2005) Article DOI: 10.1021/jm058235e BindingDB Entry DOI: 10.7270/Q20R9P0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366521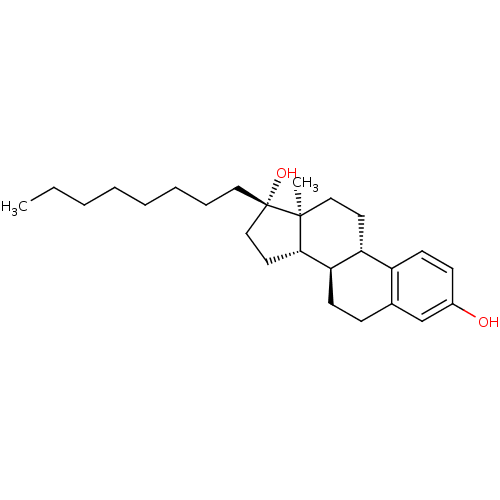 (CHEMBL1627427) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Curated by ChEMBL | Assay Description Estrone sulfatase activity against homogenized human JEG-3 cells was determined by measuring the [3H]E1 obtained from [3H]E1S | Bioorg Med Chem Lett 8: 1891-6 (1999) BindingDB Entry DOI: 10.7270/Q2PV6KWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366521 (CHEMBL1627427) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50366344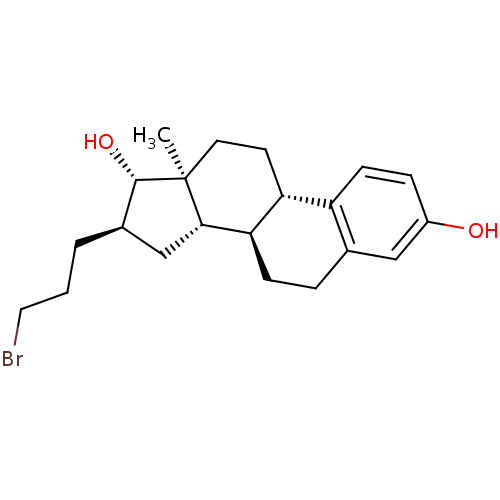 (CHEMBL1627450) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the inhibition of 17-beta-hydroxysteroid dehydrogenase type 1(17-beta-HSD type 1) | Bioorg Med Chem Lett 4: 2129-2132 (1994) Article DOI: 10.1016/S0960-894X(01)80115-3 BindingDB Entry DOI: 10.7270/Q2JW8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369742 (CHEMBL1627627) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Laval University Medical Center (CHUL) Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase activity of JEG-3 cells | J Med Chem 43: 4465-78 (2000) BindingDB Entry DOI: 10.7270/Q2MC90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 81 total ) | Next | Last >> |