

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 recombinant aspartic protease | Bioorg Med Chem 16: 902-8 (2008) Article DOI: 10.1016/j.bmc.2007.10.020 BindingDB Entry DOI: 10.7270/Q2CZ381K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50213021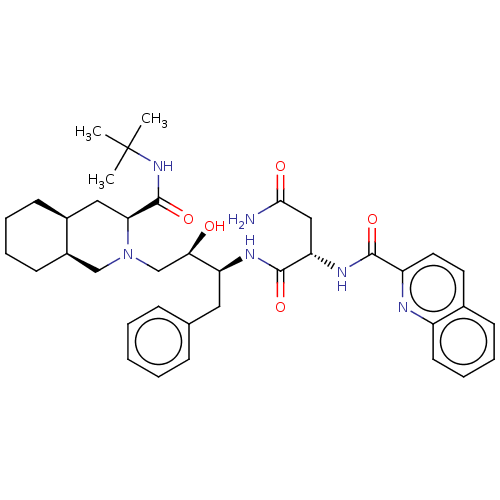 (CHEBI:63621 | Fortovase | Invirase | Ro-31-8959 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 protease assessed as hydrolysis of fluorogenic substrate | J Med Chem 53: 1451-7 (2010) Article DOI: 10.1021/jm900846f BindingDB Entry DOI: 10.7270/Q2P271XP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481430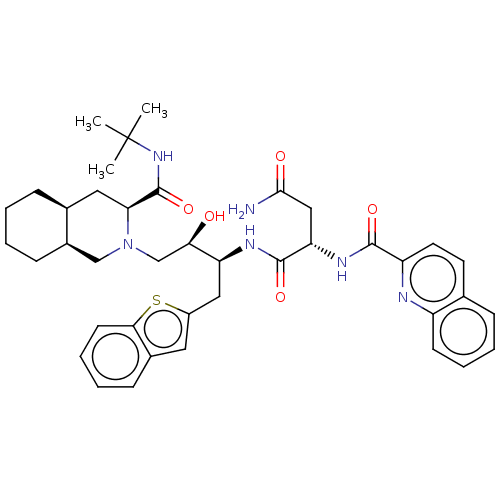 (CHEMBL591934) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 protease assessed as hydrolysis of fluorogenic substrate | J Med Chem 53: 1451-7 (2010) Article DOI: 10.1021/jm900846f BindingDB Entry DOI: 10.7270/Q2P271XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481429 (CHEMBL577443) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 protease assessed as hydrolysis of fluorogenic substrate | J Med Chem 53: 1451-7 (2010) Article DOI: 10.1021/jm900846f BindingDB Entry DOI: 10.7270/Q2P271XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50061306 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 protease assessed as hydrolysis of fluorogenic substrate | J Med Chem 53: 1451-7 (2010) Article DOI: 10.1021/jm900846f BindingDB Entry DOI: 10.7270/Q2P271XP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481435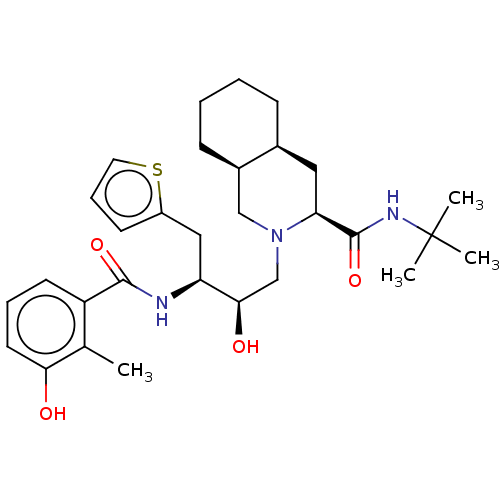 (CHEMBL606392) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 protease assessed as hydrolysis of fluorogenic substrate | J Med Chem 53: 1451-7 (2010) Article DOI: 10.1021/jm900846f BindingDB Entry DOI: 10.7270/Q2P271XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481434 (CHEMBL577638) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 protease assessed as hydrolysis of fluorogenic substrate | J Med Chem 53: 1451-7 (2010) Article DOI: 10.1021/jm900846f BindingDB Entry DOI: 10.7270/Q2P271XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481433 (CHEMBL591933) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 protease assessed as hydrolysis of fluorogenic substrate | J Med Chem 53: 1451-7 (2010) Article DOI: 10.1021/jm900846f BindingDB Entry DOI: 10.7270/Q2P271XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481431 (CHEMBL578278) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 protease assessed as hydrolysis of fluorogenic substrate | J Med Chem 53: 1451-7 (2010) Article DOI: 10.1021/jm900846f BindingDB Entry DOI: 10.7270/Q2P271XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50373904 (CHEMBL405969) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 recombinant aspartic protease | Bioorg Med Chem 16: 902-8 (2008) Article DOI: 10.1016/j.bmc.2007.10.020 BindingDB Entry DOI: 10.7270/Q2CZ381K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50373905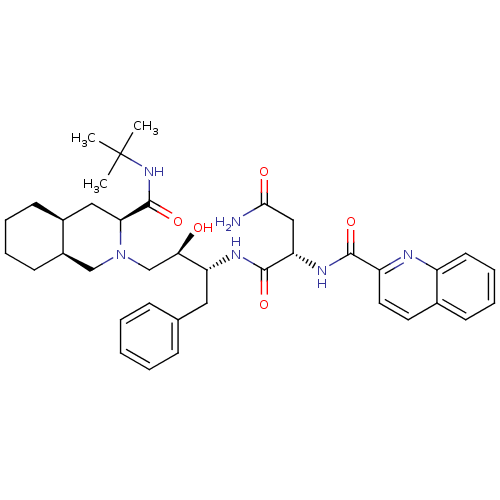 (CHEMBL438376) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 483 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ La Sapienza Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 recombinant aspartic protease | Bioorg Med Chem 16: 902-8 (2008) Article DOI: 10.1016/j.bmc.2007.10.020 BindingDB Entry DOI: 10.7270/Q2CZ381K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481428 (CHEMBL578874) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 protease assessed as hydrolysis of fluorogenic substrate | J Med Chem 53: 1451-7 (2010) Article DOI: 10.1021/jm900846f BindingDB Entry DOI: 10.7270/Q2P271XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481426 (CHEMBL578053) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 protease assessed as hydrolysis of fluorogenic substrate | J Med Chem 53: 1451-7 (2010) Article DOI: 10.1021/jm900846f BindingDB Entry DOI: 10.7270/Q2P271XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481427 (CHEMBL577017) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 protease assessed as hydrolysis of fluorogenic substrate | J Med Chem 53: 1451-7 (2010) Article DOI: 10.1021/jm900846f BindingDB Entry DOI: 10.7270/Q2P271XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481432 (CHEMBL597982) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV1 protease assessed as hydrolysis of fluorogenic substrate | J Med Chem 53: 1451-7 (2010) Article DOI: 10.1021/jm900846f BindingDB Entry DOI: 10.7270/Q2P271XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||