

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM412734 (N-(3-fluoropyridin-4-yl)-2-(6-methylpyridin-2-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay | Bioorg Med Chem 26: 1026-1034 (2018) Article DOI: 10.1016/j.bmc.2018.01.014 BindingDB Entry DOI: 10.7270/Q27M0BHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50454871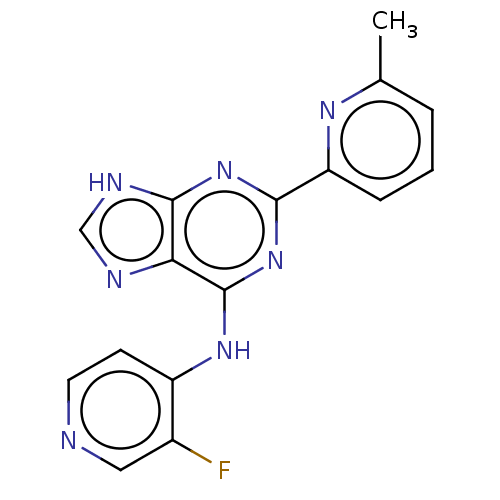 (CHEMBL4209835) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay | Bioorg Med Chem 26: 1026-1034 (2018) Article DOI: 10.1016/j.bmc.2018.01.014 BindingDB Entry DOI: 10.7270/Q27M0BHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM412755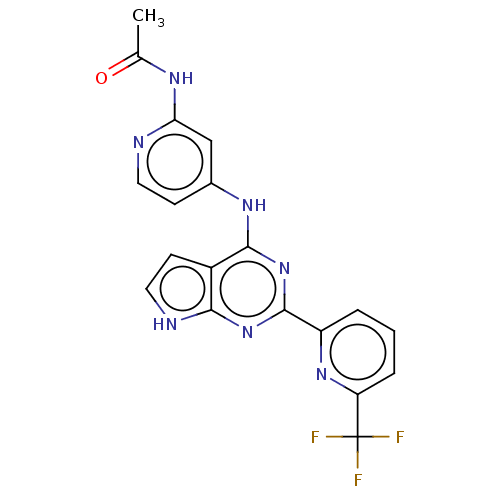 (N-(4-((2-(6-(trifluoromethyl)pyridin-2-yl)-7H-pyrr...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay | Bioorg Med Chem 26: 1026-1034 (2018) Article DOI: 10.1016/j.bmc.2018.01.014 BindingDB Entry DOI: 10.7270/Q27M0BHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM412745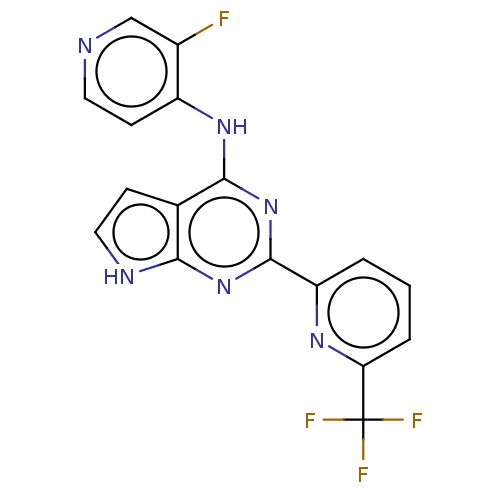 (N-(3-fluoropyridin-4-yl)-2-(6-(trifluoromethyl)pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PDB UniChem | PDB Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged TGFbetaR1 T204D mutant expressed in Sf9 insect cells after 1 hr by HTRF assay | Bioorg Med Chem 26: 1026-1034 (2018) Article DOI: 10.1016/j.bmc.2018.01.014 BindingDB Entry DOI: 10.7270/Q27M0BHV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM282825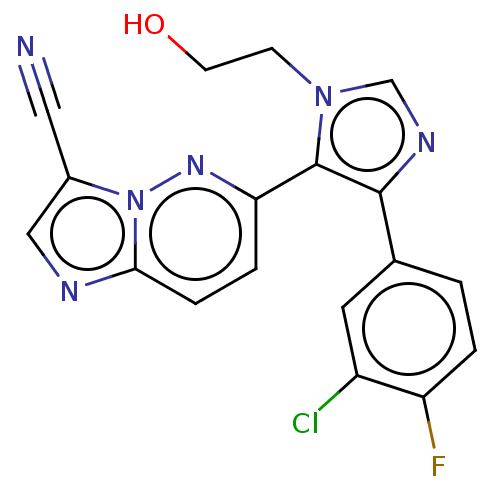 (6-(4-(3-chloro-4-fluorophenyl)-1-(2-hydroxyethyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Inhibition of TGFBR1 in human whole blood assessed as apparent inhibition constant by measuring reduction in TGFbeta-induced SMAD phosphorylation | ACS Med Chem Lett 11: 172-178 (2020) Article DOI: 10.1021/acsmedchemlett.9b00552 BindingDB Entry DOI: 10.7270/Q2MP56K3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| TGF-beta receptor type-1 (Mus musculus) | BDBM282825 (6-(4-(3-chloro-4-fluorophenyl)-1-(2-hydroxyethyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development Curated by ChEMBL | Assay Description Inhibition of TGFBR1 in mouse whole blood assessed as apparent inhibition constant by measuring reduction in TGFbeta-induced SMAD phosphorylation | ACS Med Chem Lett 11: 172-178 (2020) Article DOI: 10.1021/acsmedchemlett.9b00552 BindingDB Entry DOI: 10.7270/Q2MP56K3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR2 | J Med Chem 49: 2143-6 (2006) Article DOI: 10.1021/jm051106d BindingDB Entry DOI: 10.7270/Q2WW7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR2 | J Med Chem 49: 2143-6 (2006) Article DOI: 10.1021/jm051106d BindingDB Entry DOI: 10.7270/Q2WW7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Mus musculus) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Flk1 | J Med Chem 49: 2143-6 (2006) Article DOI: 10.1021/jm051106d BindingDB Entry DOI: 10.7270/Q2WW7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50184807 ((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against VEGFR1 | J Med Chem 49: 2143-6 (2006) Article DOI: 10.1021/jm051106d BindingDB Entry DOI: 10.7270/Q2WW7H7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249161 (US9453048, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249163 (US9453048, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249167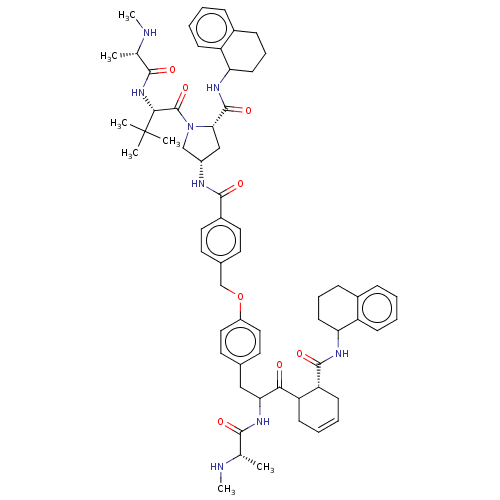 (US9453048, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249169 (US9453048, 11 | US9453048, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50168389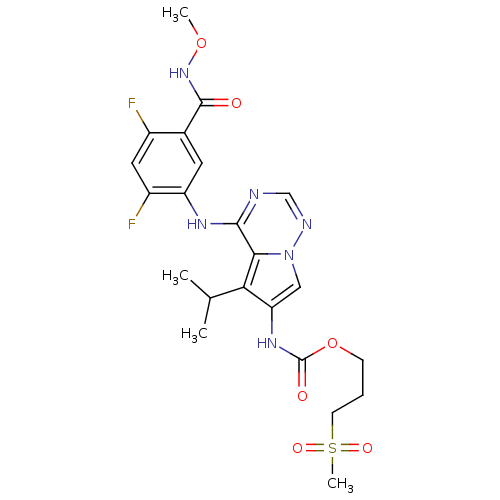 (CHEMBL195218 | [4-(2,4-Difluoro-5-methoxycarbamoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human vascular endothelial growth factor receptor 2 (VEGFR-2) | J Med Chem 48: 3991-4008 (2005) Article DOI: 10.1021/jm0501275 BindingDB Entry DOI: 10.7270/Q2KP81QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249174 (US9453048, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249172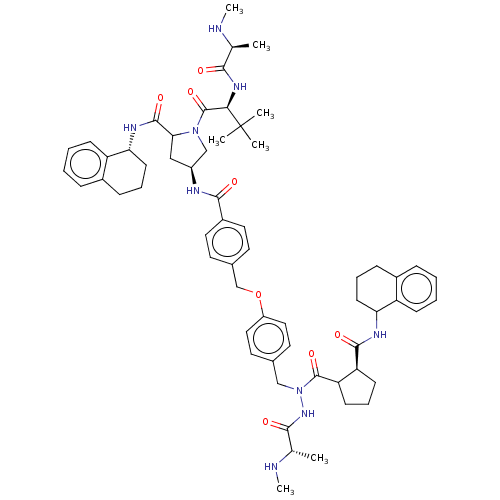 (US9453048, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249162 (US9453048, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249168 (US9453048, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249160 (US9453048, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249166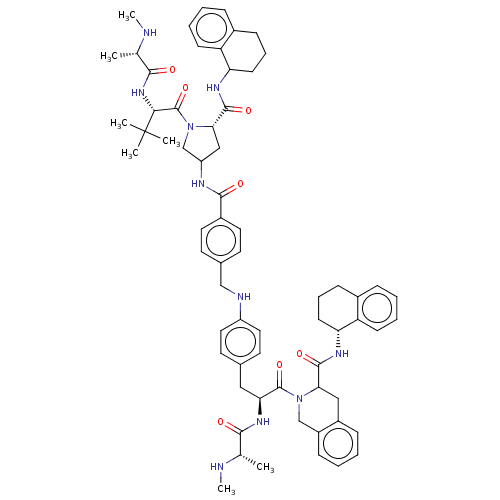 (US9453048, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249165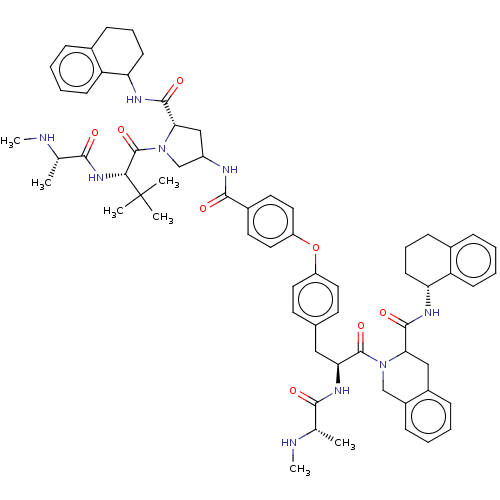 (US9453048, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249171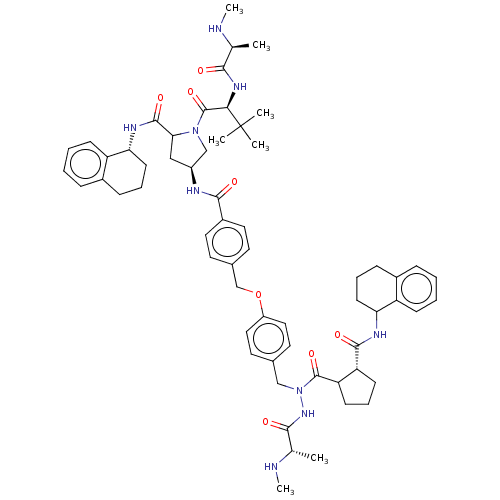 (US9453048, 13 | US9453048, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249164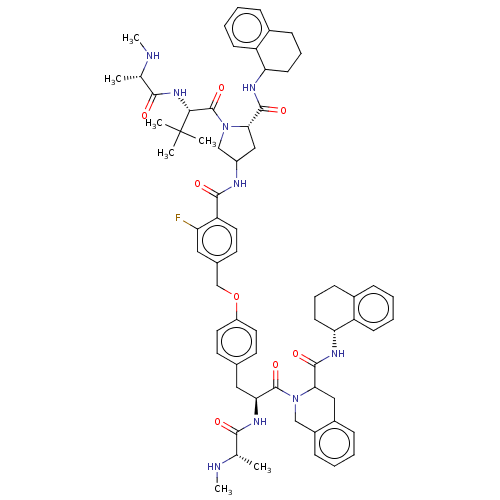 (US9453048, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249178 (US9453048, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM406730 (N-[4-({2-[6-(difluoromethyl)pyridin-2-yl]pyrrolo[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM406730 (N-[4-({2-[6-(difluoromethyl)pyridin-2-yl]pyrrolo[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249177 (US9453048, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [124-240] (Homo sapiens (Human)) | BDBM249163 (US9453048, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in white, flat-bottom, 384-well ProxiPlates (Perkin Elmer). The final assay volume was 10 μL prepared from additions of Hi... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249176 (US9453048, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249171 (US9453048, 13 | US9453048, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249169 (US9453048, 11 | US9453048, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM412768 (N-(3-fluoropyridin-4-yl)-5-methyl-2-(6-methylpyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myer Squibb Company US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10399987 (2019) BindingDB Entry DOI: 10.7270/Q2222X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM406951 (1-({[2-(5-fluoropyridin-2-yl)-4-[(3-fluoropyridin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM412735 (2-(6-(difluoromethyl)pyridin-2-yl)-N-(3-fluoropyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myer Squibb Company US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10399987 (2019) BindingDB Entry DOI: 10.7270/Q2222X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM407009 (N-(4-{[5-(morpholin-4-ylmethyl)-2-(pyridin-2-yl)py...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM407012 ((3-{[({4-[(3-fluoropyridin-4-yl)amino]-2-(pyridin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM406793 (N-{5-[(4,4-difluoropiperidin-1-yl)methyl]-2-(6-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM412743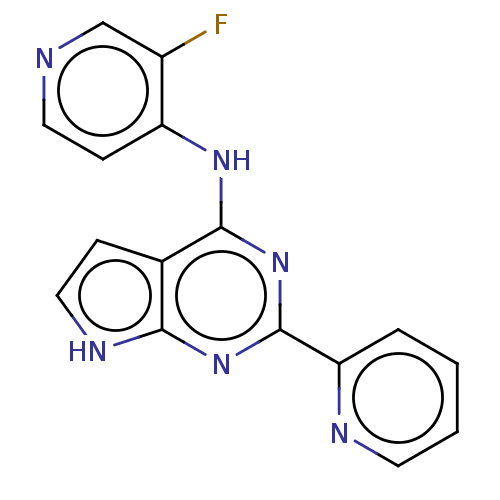 (N-(3-fluoropyridin-4-yl)-2-(pyridin-2-yl)-7H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myer Squibb Company US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10399987 (2019) BindingDB Entry DOI: 10.7270/Q2222X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM406957 (3-fluoro-N-(5-{[(1-methylcyclobutyl)amino]methyl}-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM249175 (US9453048, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of N-His-Tb-BIR3(241-356,... | US Patent US9453048 (2016) BindingDB Entry DOI: 10.7270/Q2057DV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [T204D] (Homo sapiens (Human)) | BDBM283066 (6-(1-(1-cyanocyclopropyl)-4-(4-fluorophenyl)-1H- i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin at Madison | Assay Description Assays for the compounds reported below were conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGF-βR1 T204D or... | J Med Chem 51: 7243-52 (2008) BindingDB Entry DOI: 10.7270/Q2B85BFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2/E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM312982 (US9605022, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corporation US Patent | Assay Description Assays were performed in black, flat-bottom, 384-well plates. The final assay volume was 50 μL prepared from additions of His-BIR2-3 (125-356, C... | US Patent US9605022 (2017) BindingDB Entry DOI: 10.7270/Q2959KMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM283066 (6-(1-(1-cyanocyclopropyl)-4-(4-fluorophenyl)-1H- i...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assays for the compounds reported below were conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGF-βR1 T204D or... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM407043 (2-[(3R)-3-hydroxypyrrolidin-1-yl]-N-[4-({2-[6-(tri...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM283066 (6-(1-(1-cyanocyclopropyl)-4-(4-fluorophenyl)-1H- i...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rigel Pharmaceuticals, Inc.; Bristol-Myers Squibb Company US Patent | Assay Description Assays for the compounds reported below were conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGF-βR1 T204D or... | US Patent US9884868 (2018) BindingDB Entry DOI: 10.7270/Q20P122M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM407051 (N-[4-({2-[6-(trifluoromethyl)pyridin-2-yl]pyrrolo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM412766 (N-(4-((2-(6-(difluoromethyl)pyridin-2-yl)-7H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myer Squibb Company US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10399987 (2019) BindingDB Entry DOI: 10.7270/Q2222X4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM406787 (3-chloro-N-[2-(6-fluoropyridin-2-yl)pyrrolo[2,1-f]...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. US Patent | Assay Description Assays are conducted in 1536-well plates and 2 mL reactions are prepared from addition of HIS-TGFβR1 T204D or HIS-TGFβR2 WT, anti-HIS detec... | US Patent US10336761 (2019) BindingDB Entry DOI: 10.7270/Q2M90C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of lck inase | J Med Chem 47: 6658-61 (2004) Article DOI: 10.1021/jm049486a BindingDB Entry DOI: 10.7270/Q2ZG6RRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4170 total ) | Next | Last >> |