 Found 46 hits with Last Name = 'bouchet' and Initial = 's'
Found 46 hits with Last Name = 'bouchet' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50525406
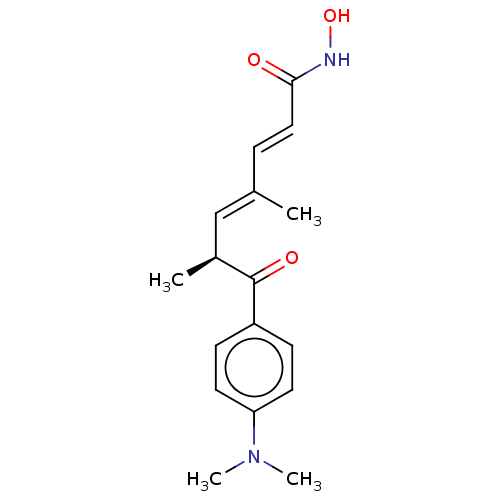
(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using fluorogenic HDAC substrate measured after 10 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate measured after 15 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using fluorogenic HDAC substrate measured after 15 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using fluorogenic HDAC substrate measured after 30 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using fluorogenic HDAC substrate measured after 45 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50525404
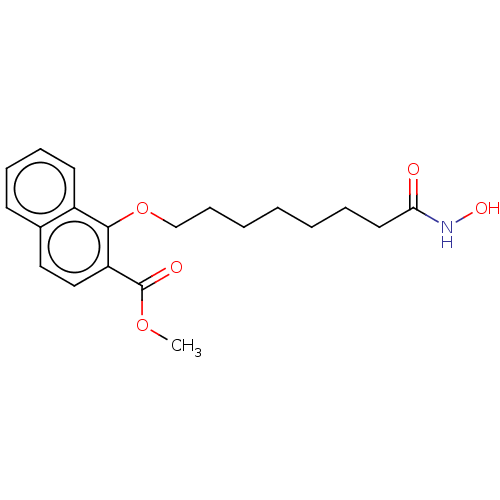
(CHEMBL4563988)Show InChI InChI=1S/C20H25NO5/c1-25-20(23)17-13-12-15-9-6-7-10-16(15)19(17)26-14-8-4-2-3-5-11-18(22)21-24/h6-7,9-10,12-13,24H,2-5,8,11,14H2,1H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using fluorogenic HDAC substrate measured after 30 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using fluorogenic HDAC substrate measured after 60 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 using fluorogenic HDAC substrate class 2a measured after 45 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50525404

(CHEMBL4563988)Show InChI InChI=1S/C20H25NO5/c1-25-20(23)17-13-12-15-9-6-7-10-16(15)19(17)26-14-8-4-2-3-5-11-18(22)21-24/h6-7,9-10,12-13,24H,2-5,8,11,14H2,1H3,(H,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using fluorogenic HDAC substrate measured after 10 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50525404

(CHEMBL4563988)Show InChI InChI=1S/C20H25NO5/c1-25-20(23)17-13-12-15-9-6-7-10-16(15)19(17)26-14-8-4-2-3-5-11-18(22)21-24/h6-7,9-10,12-13,24H,2-5,8,11,14H2,1H3,(H,21,22) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using fluorogenic HDAC substrate measured after 45 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50525404

(CHEMBL4563988)Show InChI InChI=1S/C20H25NO5/c1-25-20(23)17-13-12-15-9-6-7-10-16(15)19(17)26-14-8-4-2-3-5-11-18(22)21-24/h6-7,9-10,12-13,24H,2-5,8,11,14H2,1H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using fluorogenic HDAC substrate measured after 60 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC5 using fluorogenic HDAC substrate class 2a measured after 30 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50115661
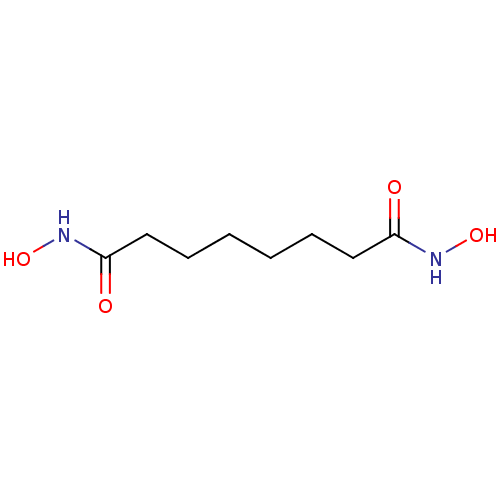
(CHEMBL320497 | N,8-dihydroxy-8-(hydroxyamino)octan...)Show InChI InChI=1S/C8H16N2O4/c11-7(9-13)5-3-1-2-4-6-8(12)10-14/h13-14H,1-6H2,(H,9,11)(H,10,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 using fluorogenic HDAC substrate class 2a measured after 30 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50499957
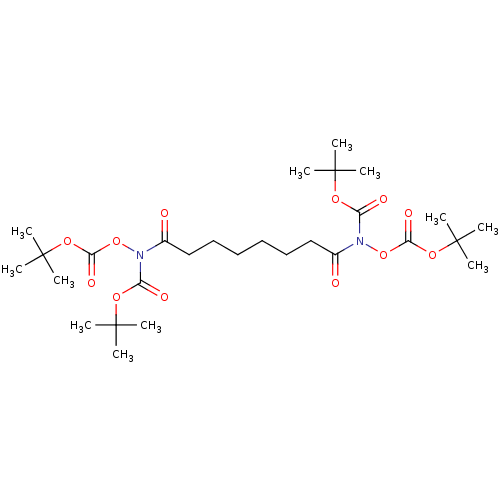
(CHEMBL3739529)Show SMILES CC(C)(C)OC(=O)ON(C(=O)CCCCCCC(=O)N(OC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)C(=O)OC(C)(C)C Show InChI InChI=1S/C28H48N2O12/c1-25(2,3)37-21(33)29(41-23(35)39-27(7,8)9)19(31)17-15-13-14-16-18-20(32)30(22(34)38-26(4,5)6)42-24(36)40-28(10,11)12/h13-18H2,1-12H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50525404

(CHEMBL4563988)Show InChI InChI=1S/C20H25NO5/c1-25-20(23)17-13-12-15-9-6-7-10-16(15)19(17)26-14-8-4-2-3-5-11-18(22)21-24/h6-7,9-10,12-13,24H,2-5,8,11,14H2,1H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using fluorogenic HDAC substrate measured after 15 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50525404

(CHEMBL4563988)Show InChI InChI=1S/C20H25NO5/c1-25-20(23)17-13-12-15-9-6-7-10-16(15)19(17)26-14-8-4-2-3-5-11-18(22)21-24/h6-7,9-10,12-13,24H,2-5,8,11,14H2,1H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate measured after 15 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50525406

(CHEMBL164868)Show SMILES C[C@@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 using fluorogenic HDAC substrate class 2a measured after 30 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50328678
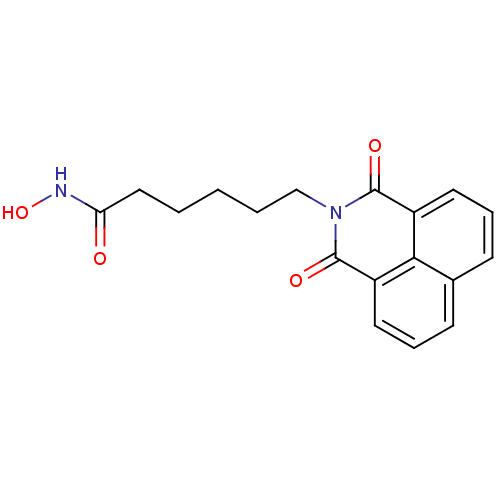
(6-(1,3-Dioxo-1H,3H-benzo[de]isoquinolin-2-yl)-hexa...)Show InChI InChI=1S/C18H18N2O4/c21-15(19-24)10-2-1-3-11-20-17(22)13-8-4-6-12-7-5-9-14(16(12)13)18(20)23/h4-9,24H,1-3,10-11H2,(H,19,21) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC11 using fluorogenic HDAC substrate class 2a measured after 30 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50115661

(CHEMBL320497 | N,8-dihydroxy-8-(hydroxyamino)octan...)Show InChI InChI=1S/C8H16N2O4/c11-7(9-13)5-3-1-2-4-6-8(12)10-14/h13-14H,1-6H2,(H,9,11)(H,10,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50525404

(CHEMBL4563988)Show InChI InChI=1S/C20H25NO5/c1-25-20(23)17-13-12-15-9-6-7-10-16(15)19(17)26-14-8-4-2-3-5-11-18(22)21-24/h6-7,9-10,12-13,24H,2-5,8,11,14H2,1H3,(H,21,22) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC11 using fluorogenic HDAC substrate class 2a measured after 30 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-1
(Homo sapiens (Human)) | BDBM50179360

(CHEMBL3040216)Show SMILES CC1=CCC(=C\C1=N\C(=O)C1=CC=C\C(C1)=N/C(=O)/N=C1/CC(=CC=C1)C(=O)\N=C1\CC(=CC=C1C)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O |c:4,13,24,26,34,36,45,72,t:1,11| Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-11,15-16,20-24H,12-14,17-19H2,1-2H3,(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)/b52-31+,53-32+,54-37+,55-38+,56-39-,57-40- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sirtuin 1 using fluorogenic HDAC substrate measured after 20 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50115661

(CHEMBL320497 | N,8-dihydroxy-8-(hydroxyamino)octan...)Show InChI InChI=1S/C8H16N2O4/c11-7(9-13)5-3-1-2-4-6-8(12)10-14/h13-14H,1-6H2,(H,9,11)(H,10,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50179360

(CHEMBL3040216)Show SMILES CC1=CCC(=C\C1=N\C(=O)C1=CC=C\C(C1)=N/C(=O)/N=C1/CC(=CC=C1)C(=O)\N=C1\CC(=CC=C1C)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O |c:4,13,24,26,34,36,45,72,t:1,11| Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-11,15-16,20-24H,12-14,17-19H2,1-2H3,(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)/b52-31+,53-32+,54-37+,55-38+,56-39-,57-40- | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sirtuin 2 using fluoro-lysine sirtuin 2 deacetylase substrate measured after 60 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50499957

(CHEMBL3739529)Show SMILES CC(C)(C)OC(=O)ON(C(=O)CCCCCCC(=O)N(OC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)C(=O)OC(C)(C)C Show InChI InChI=1S/C28H48N2O12/c1-25(2,3)37-21(33)29(41-23(35)39-27(7,8)9)19(31)17-15-13-14-16-18-20(32)30(22(34)38-26(4,5)6)42-24(36)40-28(10,11)12/h13-18H2,1-12H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50499957

(CHEMBL3739529)Show SMILES CC(C)(C)OC(=O)ON(C(=O)CCCCCCC(=O)N(OC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)C(=O)OC(C)(C)C Show InChI InChI=1S/C28H48N2O12/c1-25(2,3)37-21(33)29(41-23(35)39-27(7,8)9)19(31)17-15-13-14-16-18-20(32)30(22(34)38-26(4,5)6)42-24(36)40-28(10,11)12/h13-18H2,1-12H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-3, mitochondrial
(Homo sapiens (Human)) | BDBM27507
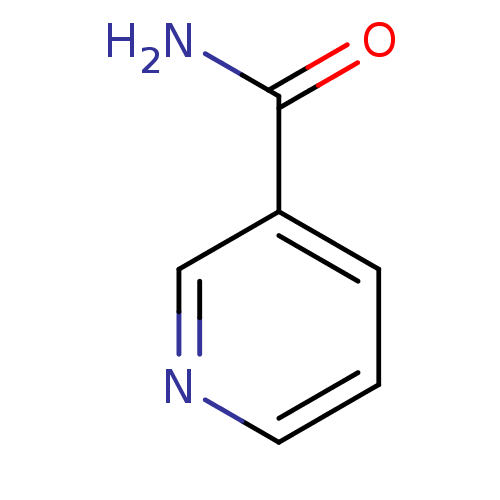
(3-Pyridinecarboxamide | CHEMBL1140 | NAM | niacina...)Show InChI InChI=1S/C6H6N2O/c7-6(9)5-2-1-3-8-4-5/h1-4H,(H2,7,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sirtuin 3 using fluoro-lysine sirtuin 2 deacetylase substrate measured after 45 mins by fluorimetry assay |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50115661

(CHEMBL320497 | N,8-dihydroxy-8-(hydroxyamino)octan...)Show InChI InChI=1S/C8H16N2O4/c11-7(9-13)5-3-1-2-4-6-8(12)10-14/h13-14H,1-6H2,(H,9,11)(H,10,12) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50499957

(CHEMBL3739529)Show SMILES CC(C)(C)OC(=O)ON(C(=O)CCCCCCC(=O)N(OC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)C(=O)OC(C)(C)C Show InChI InChI=1S/C28H48N2O12/c1-25(2,3)37-21(33)29(41-23(35)39-27(7,8)9)19(31)17-15-13-14-16-18-20(32)30(22(34)38-26(4,5)6)42-24(36)40-28(10,11)12/h13-18H2,1-12H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) by fluorimetric assay |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 in human HeLa cells assessed as inhibition of tubulin deacetylation incubated for overnight by cELISA |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human MeT-5A cells assessed as increase in transfected YFP fused histone H3 acetylation treated at 8 hrs after YFP fused H3 tra... |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50499957

(CHEMBL3739529)Show SMILES CC(C)(C)OC(=O)ON(C(=O)CCCCCCC(=O)N(OC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)C(=O)OC(C)(C)C Show InChI InChI=1S/C28H48N2O12/c1-25(2,3)37-21(33)29(41-23(35)39-27(7,8)9)19(31)17-15-13-14-16-18-20(32)30(22(34)38-26(4,5)6)42-24(36)40-28(10,11)12/h13-18H2,1-12H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 in human HeLa cells assessed as inhibition of tubulin deacetylation incubated for overnight by cELISA |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
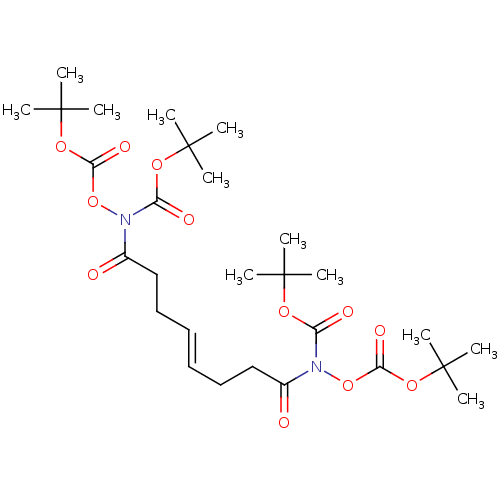
(Homo sapiens (Human)) | BDBM50499958
(CHEMBL3740591)Show SMILES CC(C)(C)OC(=O)ON(C(=O)CC\C=C\CCC(=O)N(OC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)C(=O)OC(C)(C)C Show InChI InChI=1S/C28H46N2O12/c1-25(2,3)37-21(33)29(41-23(35)39-27(7,8)9)19(31)17-15-13-14-16-18-20(32)30(22(34)38-26(4,5)6)42-24(36)40-28(10,11)12/h13-14H,15-18H2,1-12H3/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 in human HeLa cells assessed as inhibition of tubulin deacetylation incubated for overnight by cELISA |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50115661

(CHEMBL320497 | N,8-dihydroxy-8-(hydroxyamino)octan...)Show InChI InChI=1S/C8H16N2O4/c11-7(9-13)5-3-1-2-4-6-8(12)10-14/h13-14H,1-6H2,(H,9,11)(H,10,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 in human HeLa cells assessed as inhibition of tubulin deacetylation incubated for overnight by cELISA |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
University of Geneva
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 in human HeLa cells assessed as inhibition of tubulin deacetylation incubated for overnight by cELISA |
Bioorg Med Chem Lett 26: 154-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.011
BindingDB Entry DOI: 10.7270/Q2BK1GCP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50525404

(CHEMBL4563988)Show InChI InChI=1S/C20H25NO5/c1-25-20(23)17-13-12-15-9-6-7-10-16(15)19(17)26-14-8-4-2-3-5-11-18(22)21-24/h6-7,9-10,12-13,24H,2-5,8,11,14H2,1H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.84E+3 | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human MeT-5A cells assessed as increase in transfected YFP fused histone H3 acetylation treated at 8 hrs after YFP fused H3 tra... |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50525405
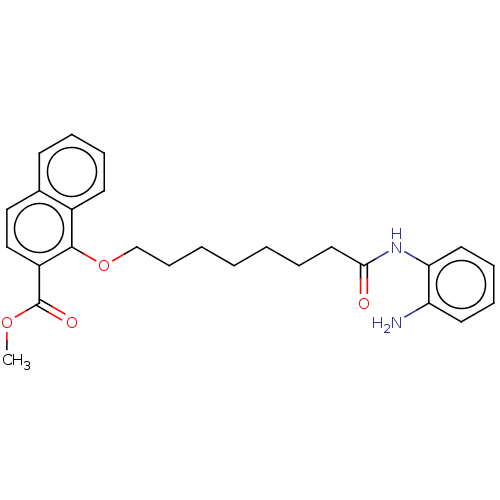
(CHEMBL4516678)Show SMILES COC(=O)c1ccc2ccccc2c1OCCCCCCCC(=O)Nc1ccccc1N Show InChI InChI=1S/C26H30N2O4/c1-31-26(30)21-17-16-19-11-6-7-12-20(19)25(21)32-18-10-4-2-3-5-15-24(29)28-23-14-9-8-13-22(23)27/h6-9,11-14,16-17H,2-5,10,15,18,27H2,1H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.14E+3 | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human MeT-5A cells assessed as increase in transfected YFP fused histone H3 acetylation treated at 8 hrs after YFP fused H3 tra... |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM19422

(4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...)Show InChI InChI=1S/C15H15N3O2/c1-10(19)17-12-8-6-11(7-9-12)15(20)18-14-5-3-2-4-13(14)16/h2-9H,16H2,1H3,(H,17,19)(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a |
UMR CNRS 7285
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human MeT-5A cells assessed as increase in transfected YFP fused histone H3 acetylation treated at 8 hrs after YFP fused H3 tra... |
ACS Med Chem Lett 10: 863-868 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00440
BindingDB Entry DOI: 10.7270/Q23J3HDS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data