

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50478518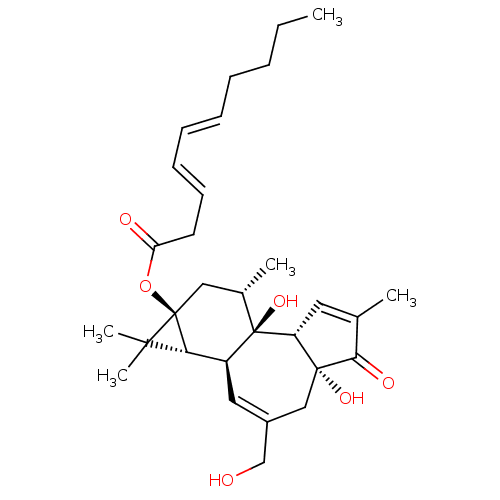 (CHEMBL514017) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Displacement of [3H]PDBu from rat brain membrane PKC | J Nat Prod 58: 769-72 (1995) Article DOI: 10.1021/np50119a020 BindingDB Entry DOI: 10.7270/Q2NZ8BF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50090045 ((2,3-Dihydroxy-4-methoxy-phenyl)-(3,4,5-trimethoxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization | J Med Chem 43: 2731-7 (2000) BindingDB Entry DOI: 10.7270/Q2SX6CFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50005480 ((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization | J Med Chem 43: 2731-7 (2000) BindingDB Entry DOI: 10.7270/Q2SX6CFQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50090044 ((Z)-2',3'-dihydroxy-3,4,4',5-tetramethoxystilbene ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization | J Med Chem 43: 2731-7 (2000) BindingDB Entry DOI: 10.7270/Q2SX6CFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50005480 ((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description The compound was tested for the concentration to inhibit 50% of tubulin polymerization. | J Med Chem 41: 1688-95 (1998) Article DOI: 10.1021/jm970644q BindingDB Entry DOI: 10.7270/Q2N300QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50006677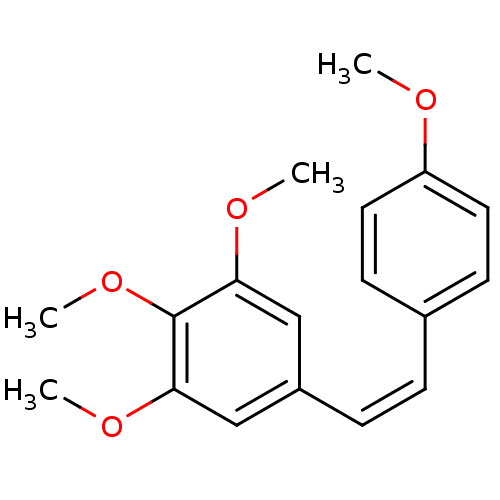 (1,2,3-Trimethoxy-5-[(Z)-2-(4-methoxy-phenyl)-vinyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description The compound was tested for the concentration to inhibit 50% of tubulin polymerization. | J Med Chem 41: 1688-95 (1998) Article DOI: 10.1021/jm970644q BindingDB Entry DOI: 10.7270/Q2N300QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50014846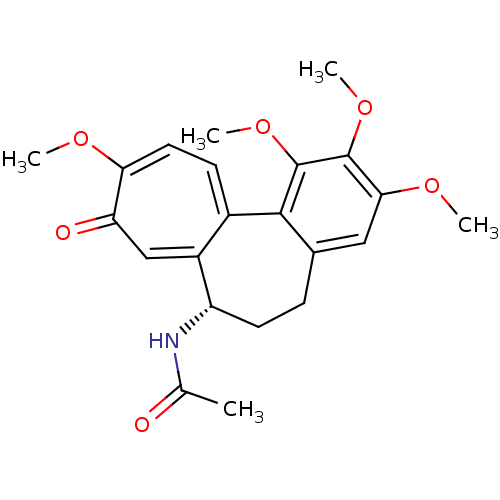 ((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for 50 % inhibition of tubulin polymerization by turbidimetric assay | Bioorg Med Chem Lett 3: 581-584 (1993) Article DOI: 10.1016/S0960-894X(01)81233-6 BindingDB Entry DOI: 10.7270/Q29G5N91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50064891 (5,7,3'-trihydroxy-3,6-4'-trimethoxyflavone | 5,7-D...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for 50 % inhibition of tubulin polymerization by turbidimetric assay | Bioorg Med Chem Lett 3: 581-584 (1993) Article DOI: 10.1016/S0960-894X(01)81233-6 BindingDB Entry DOI: 10.7270/Q29G5N91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478521 (CHEMBL465805) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by UIC assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50471653 (CHEMBL297299) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description The compound was tested for the concentration to inhibit 50% of tubulin polymerization. | J Med Chem 41: 1688-95 (1998) Article DOI: 10.1021/jm970644q BindingDB Entry DOI: 10.7270/Q2N300QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50292441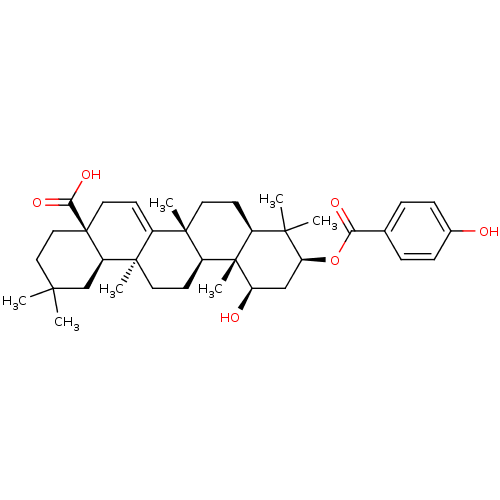 (1beta-hydroxyaleuritolic acid 3-p-hydroxybenzoate ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by UIC assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478525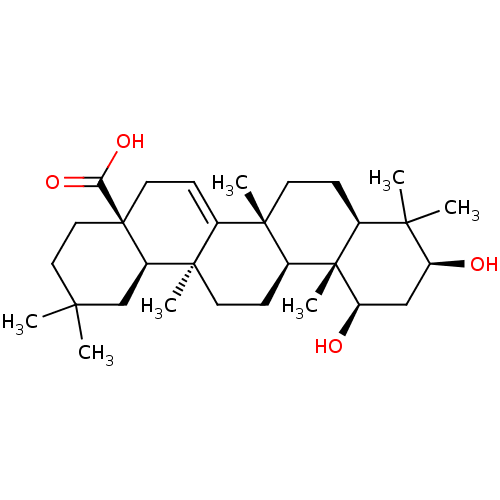 (CHEMBL465804) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by UIC assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478526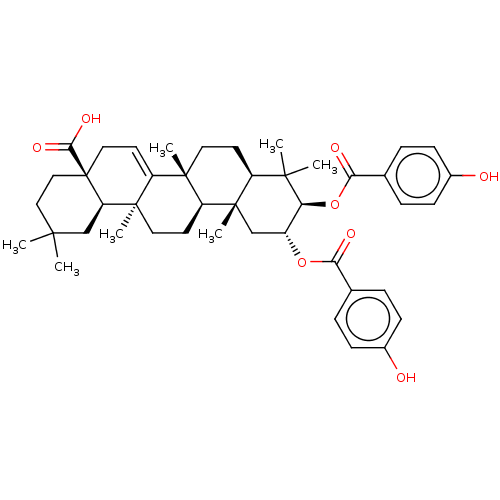 (CHEMBL448121) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by UIC assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478416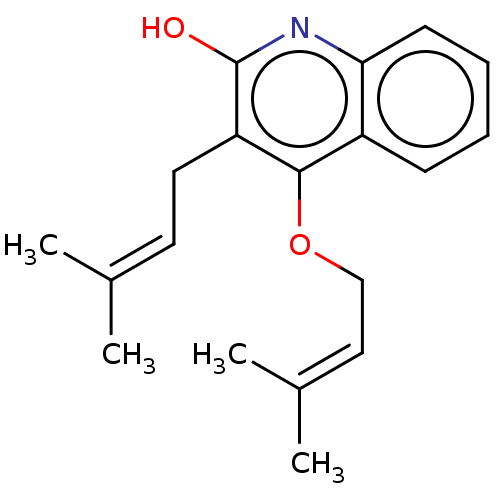 (CHEBI:65536 | CHEMBL444821) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | J Nat Prod 59: 469-71 (1996) Article DOI: 10.1021/np960250m BindingDB Entry DOI: 10.7270/Q2XK8J9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478527 (Aleuritolic Acid 3-P-Hydroxybenzoate | CHEMBL45689...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by UIC assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478523 (Aleuritolic Acid 3-P-Hydroxycinnamate | CHEMBL5033...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by UIC assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478415 (Buchapine | CHEBI:65535) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | J Nat Prod 59: 469-71 (1996) Article DOI: 10.1021/np960250m BindingDB Entry DOI: 10.7270/Q2XK8J9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478524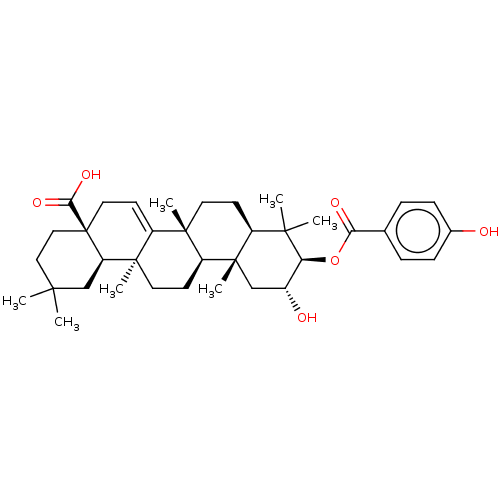 (CHEMBL450856) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by UIC assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478522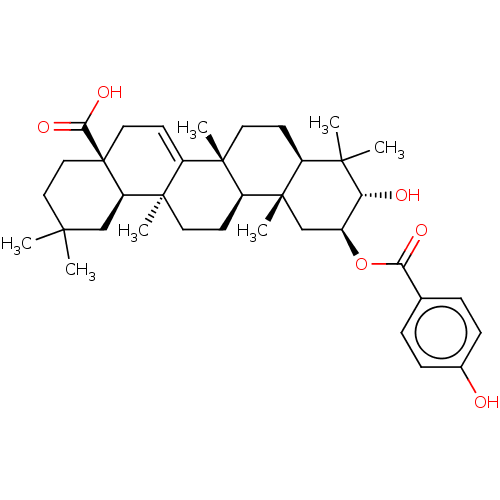 (CHEMBL454238) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by UIC assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50471654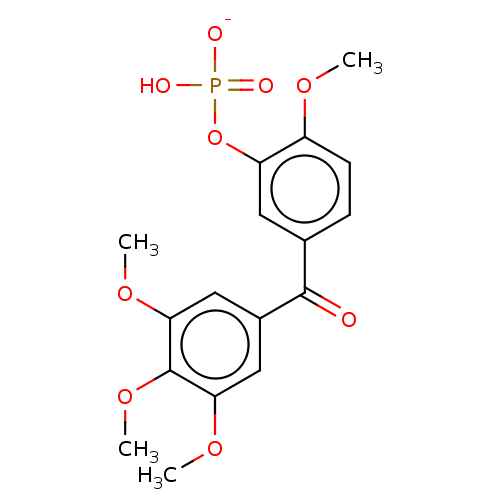 (CHEMBL44574) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description The compound was tested for the concentration to inhibit 50% of tubulin polymerization. | J Med Chem 41: 1688-95 (1998) Article DOI: 10.1021/jm970644q BindingDB Entry DOI: 10.7270/Q2N300QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478522 (CHEMBL454238) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by NCI assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50090046 (CHEMBL329267 | Phosphoric acid mono-[3-methoxy-2-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization | J Med Chem 43: 2731-7 (2000) BindingDB Entry DOI: 10.7270/Q2SX6CFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50064259 (CHEMBL289351 | Combretastatin A4 phosphate | Di-So...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization | J Med Chem 43: 2731-7 (2000) BindingDB Entry DOI: 10.7270/Q2SX6CFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478528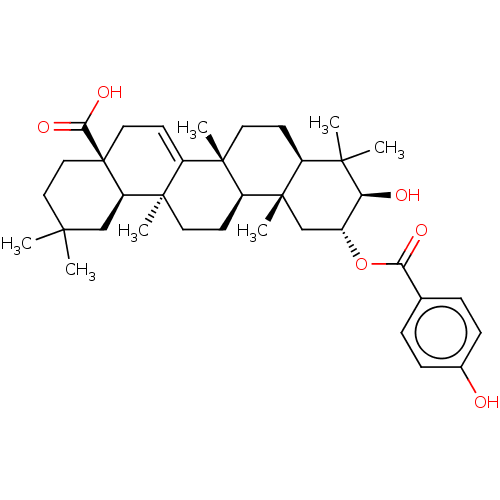 (CHEMBL451536) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by UIC assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478526 (CHEMBL448121) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by NCI assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478523 (Aleuritolic Acid 3-P-Hydroxycinnamate | CHEMBL5033...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by NCI assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50064259 (CHEMBL289351 | Combretastatin A4 phosphate | Di-So...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description The compound was tested for the concentration to inhibit 50% of tubulin polymerization. | J Med Chem 41: 1688-95 (1998) Article DOI: 10.1021/jm970644q BindingDB Entry DOI: 10.7270/Q2N300QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478521 (CHEMBL465805) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by NCI assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50292441 (1beta-hydroxyaleuritolic acid 3-p-hydroxybenzoate ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by NCI assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478528 (CHEMBL451536) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by NCI assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478524 (CHEMBL450856) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by NCI assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478527 (Aleuritolic Acid 3-P-Hydroxybenzoate | CHEMBL45689...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase by NCI assay | J Nat Prod 58: 1039-46 (1995) Article DOI: 10.1021/np50121a008 BindingDB Entry DOI: 10.7270/Q28S4SP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||