 Found 615 hits with Last Name = 'bresciani' and Initial = 'a'
Found 615 hits with Last Name = 'bresciani' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Relaxin receptor 1
(Homo sapiens (Human)) | BDBM50563230

(CHEMBL4754949)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CSSC[C@@H]2NC(=O)[C@H](CSCCSC[C@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc3cnc[nH]3)NC2=O)C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccccc2)C(=O)NC(CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC1=O)C(C)C)[C@@H](C)CC)[C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CO)C(O)=O)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)CC(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Eu3+-labelled H2 relaxin from human RXFP1 expressed in human HEK-293T cells in presence of 10 % FCS by competition binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01533
BindingDB Entry DOI: 10.7270/Q2XW4PHG |
More data for this
Ligand-Target Pair | |
Relaxin receptor 1
(Homo sapiens (Human)) | BDBM50563231

(CHEMBL4780098)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](N)C(C)C)[C@@H](C)CC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of Eu3+-labelled H2 relaxin from human RXFP1 expressed in human HEK-293T cells in presence of 10 % FCS by competition binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01533
BindingDB Entry DOI: 10.7270/Q2XW4PHG |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50461260
(CHEMBL4228926)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1ccccc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N |r| Show InChI InChI=1S/C25H29N3O4S/c1-18(2)16-22(24(30)28-25(17-26)12-14-33(31,32)15-13-25)27-23(29)21-10-8-20(9-11-21)19-6-4-3-5-7-19/h3-11,18,22H,12-16H2,1-2H3,(H,27,29)(H,28,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50461249

(CHEMBL1215628)Show SMILES CC(C)CN(NC(=O)c1ccc(CN2CCN(C)CC2)cc1)c1nc(ncc1Br)C#N Show InChI InChI=1S/C22H28BrN7O/c1-16(2)14-30(21-19(23)13-25-20(12-24)26-21)27-22(31)18-6-4-17(5-7-18)15-29-10-8-28(3)9-11-29/h4-7,13,16H,8-11,14-15H2,1-3H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461249

(CHEMBL1215628)Show SMILES CC(C)CN(NC(=O)c1ccc(CN2CCN(C)CC2)cc1)c1nc(ncc1Br)C#N Show InChI InChI=1S/C22H28BrN7O/c1-16(2)14-30(21-19(23)13-25-20(12-24)26-21)27-22(31)18-6-4-17(5-7-18)15-29-10-8-28(3)9-11-29/h4-7,13,16H,8-11,14-15H2,1-3H3,(H,27,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50461249

(CHEMBL1215628)Show SMILES CC(C)CN(NC(=O)c1ccc(CN2CCN(C)CC2)cc1)c1nc(ncc1Br)C#N Show InChI InChI=1S/C22H28BrN7O/c1-16(2)14-30(21-19(23)13-25-20(12-24)26-21)27-22(31)18-6-4-17(5-7-18)15-29-10-8-28(3)9-11-29/h4-7,13,16H,8-11,14-15H2,1-3H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50461256

(CHEMBL4228143)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(CN2CCN(C)CC2)cc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N |r| Show InChI InChI=1S/C25H37N5O4S/c1-19(2)16-22(24(32)28-25(18-26)8-14-35(33,34)15-9-25)27-23(31)21-6-4-20(5-7-21)17-30-12-10-29(3)11-13-30/h4-7,19,22H,8-17H2,1-3H3,(H,27,31)(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50461256

(CHEMBL4228143)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(CN2CCN(C)CC2)cc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N |r| Show InChI InChI=1S/C25H37N5O4S/c1-19(2)16-22(24(32)28-25(18-26)8-14-35(33,34)15-9-25)27-23(31)21-6-4-20(5-7-21)17-30-12-10-29(3)11-13-30/h4-7,19,22H,8-17H2,1-3H3,(H,27,31)(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50461260

(CHEMBL4228926)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1ccccc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N |r| Show InChI InChI=1S/C25H29N3O4S/c1-18(2)16-22(24(30)28-25(17-26)12-14-33(31,32)15-13-25)27-23(29)21-10-8-20(9-11-21)19-6-4-3-5-7-19/h3-11,18,22H,12-16H2,1-2H3,(H,27,29)(H,28,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461265
(CHEMBL4226307)Show SMILES O=C(NC1(CC1)C#N)[C@H](Cc1cccnc1)NC(=O)c1cccc(c1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C22H20N6O2/c23-14-22(6-7-22)28-21(30)19(9-15-3-2-8-24-11-15)27-20(29)17-5-1-4-16(10-17)18-12-25-26-13-18/h1-5,8,10-13,19H,6-7,9H2,(H,25,26)(H,27,29)(H,28,30)/t19-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50461248

(CHEMBL4227295)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1ccccc1)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H25N3O2/c1-16(2)14-20(22(28)26-23(15-24)12-13-23)25-21(27)19-10-8-18(9-11-19)17-6-4-3-5-7-17/h3-11,16,20H,12-14H2,1-2H3,(H,25,27)(H,26,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50175036
(CHEMBL3809599)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C12CCN(CC1)CC2)c1ncc([nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C18H11Cl2F3N2OS/c19-13-2-1-3-14(20)12(13)8-16-24-9-15(27-16)17(26)25-11-6-4-10(5-7-11)18(21,22)23/h1-7,9H,8H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged HDAC1 expressed in insect cells preincubated for 10 mins followed by addition of FLUOR DE LYS as fluoresce... |
ACS Med Chem Lett 7: 454-9 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00468
BindingDB Entry DOI: 10.7270/Q2S184FM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612447

(CHEMBL5285293) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50592391
(CHEMBL5187766) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612445

(CHEMBL5282110) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612450
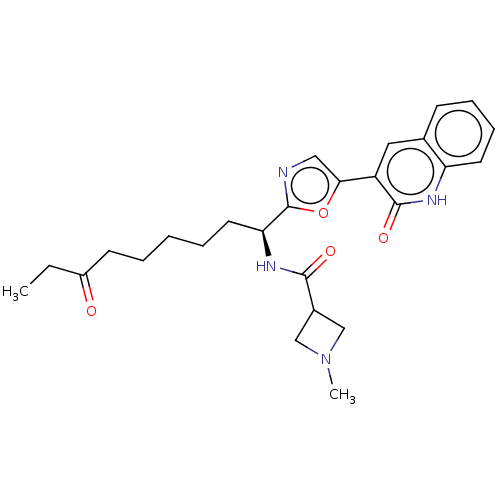
(CHEMBL5279520) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461271
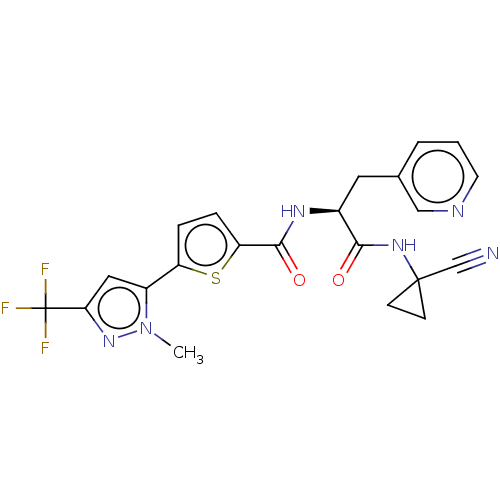
(CHEMBL4224764)Show SMILES Cn1nc(cc1-c1ccc(s1)C(=O)N[C@@H](Cc1cccnc1)C(=O)NC1(CC1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C22H19F3N6O2S/c1-31-15(10-18(30-31)22(23,24)25)16-4-5-17(34-16)20(33)28-14(9-13-3-2-8-27-11-13)19(32)29-21(12-26)6-7-21/h2-5,8,10-11,14H,6-7,9H2,1H3,(H,28,33)(H,29,32)/t14-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461272
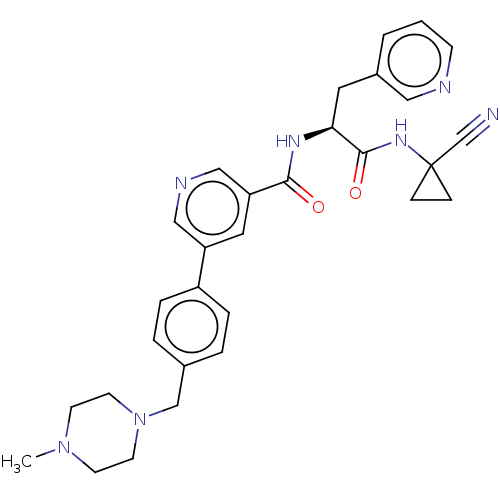
(CHEMBL4227031)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cncc(c2)C(=O)N[C@@H](Cc2cccnc2)C(=O)NC2(CC2)C#N)CC1 |r| Show InChI InChI=1S/C30H33N7O2/c1-36-11-13-37(14-12-36)20-22-4-6-24(7-5-22)25-16-26(19-33-18-25)28(38)34-27(15-23-3-2-10-32-17-23)29(39)35-30(21-31)8-9-30/h2-7,10,16-19,27H,8-9,11-15,20H2,1H3,(H,34,38)(H,35,39)/t27-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50592384
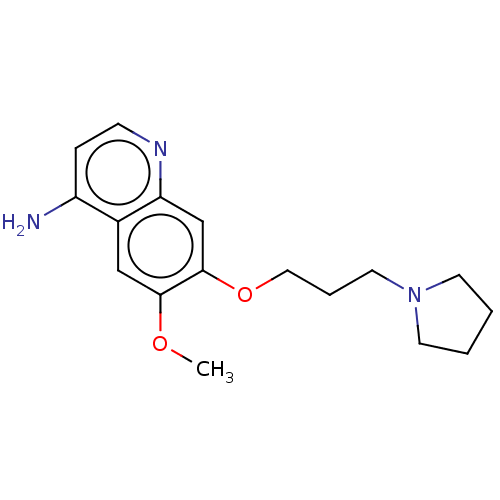
(CHEMBL5195846) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461269
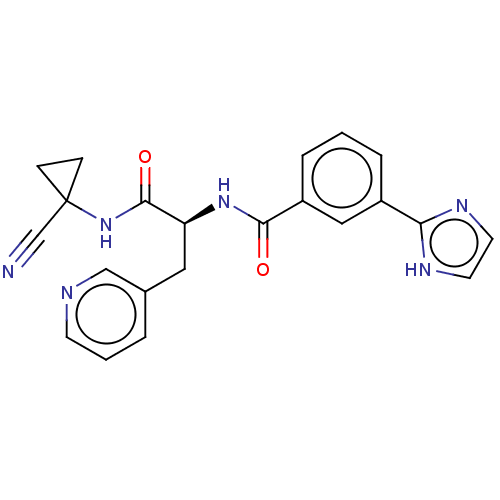
(CHEMBL4227166)Show SMILES O=C(NC1(CC1)C#N)[C@H](Cc1cccnc1)NC(=O)c1cccc(c1)-c1ncc[nH]1 |r| Show InChI InChI=1S/C22H20N6O2/c23-14-22(6-7-22)28-21(30)18(11-15-3-2-8-24-13-15)27-20(29)17-5-1-4-16(12-17)19-25-9-10-26-19/h1-5,8-10,12-13,18H,6-7,11H2,(H,25,26)(H,27,29)(H,28,30)/t18-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50612449

(CHEMBL5271471) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50446376
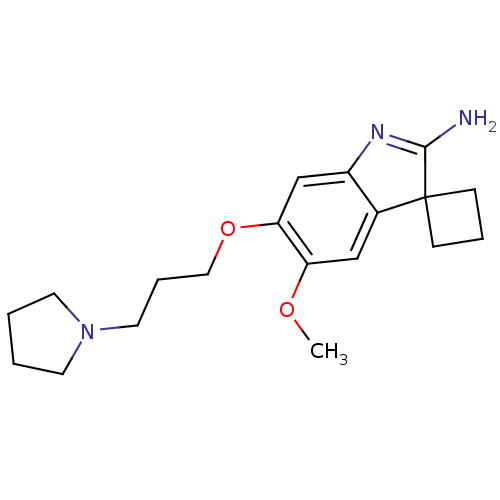
(CHEMBL3109630)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(N)C21CCC1 |t:19| Show InChI InChI=1S/C19H27N3O2/c1-23-16-12-14-15(21-18(20)19(14)6-4-7-19)13-17(16)24-11-5-10-22-8-2-3-9-22/h12-13H,2-11H2,1H3,(H2,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50352418
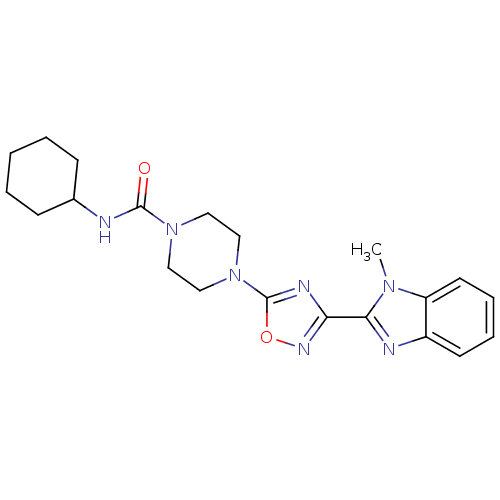
(CHEMBL1823863)Show SMILES Cn1c(nc2ccccc12)-c1noc(n1)N1CCN(CC1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C21H27N7O2/c1-26-17-10-6-5-9-16(17)23-19(26)18-24-21(30-25-18)28-13-11-27(12-14-28)20(29)22-15-7-3-2-4-8-15/h5-6,9-10,15H,2-4,7-8,11-14H2,1H3,(H,22,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis |
Bioorg Med Chem Lett 21: 5274-82 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.031
BindingDB Entry DOI: 10.7270/Q2MC90DS |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461270
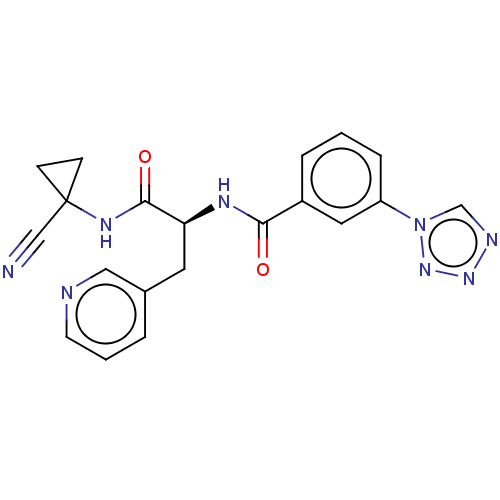
(CHEMBL4227486)Show SMILES O=C(NC1(CC1)C#N)[C@H](Cc1cccnc1)NC(=O)c1cccc(c1)-n1cnnn1 |r| Show InChI InChI=1S/C20H18N8O2/c21-12-20(6-7-20)25-19(30)17(9-14-3-2-8-22-11-14)24-18(29)15-4-1-5-16(10-15)28-13-23-26-27-28/h1-5,8,10-11,13,17H,6-7,9H2,(H,24,29)(H,25,30)/t17-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612452
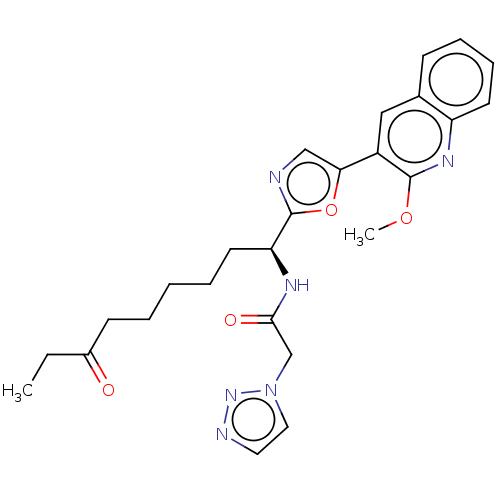
(CHEMBL5285418) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50547574
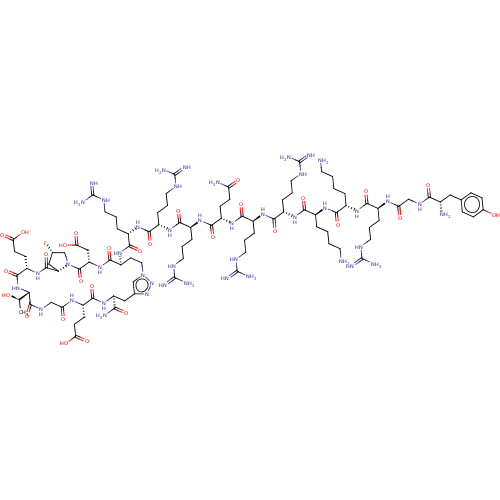
(CHEMBL4747964)Show SMILES [H][C@@]12C[C@H](F)CN1C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCn1cc(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@H](CCC(O)=O)NC2=O)[C@@H](C)O)C(N)=O)nn1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115738
BindingDB Entry DOI: 10.7270/Q2QV3R4S |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50547568

(CHEMBL4791554)Show SMILES [H][C@@]12C[C@H](F)CN1C(=O)[C@H](CC(O)=O)NC(=O)[C@]([H])(CCC(=O)NC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@H](CCC(O)=O)NC2=O)[C@@H](C)O)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115738
BindingDB Entry DOI: 10.7270/Q2QV3R4S |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50612453
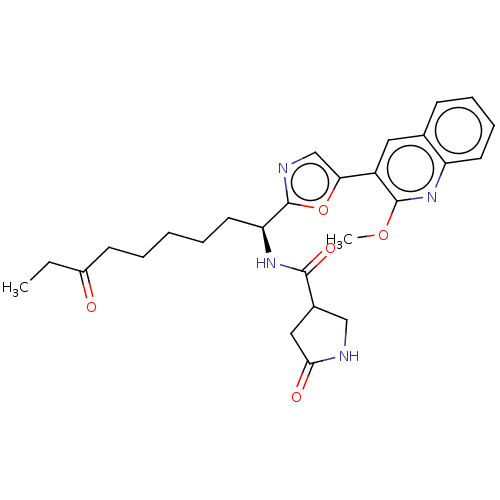
(CHEMBL5285433) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50258549
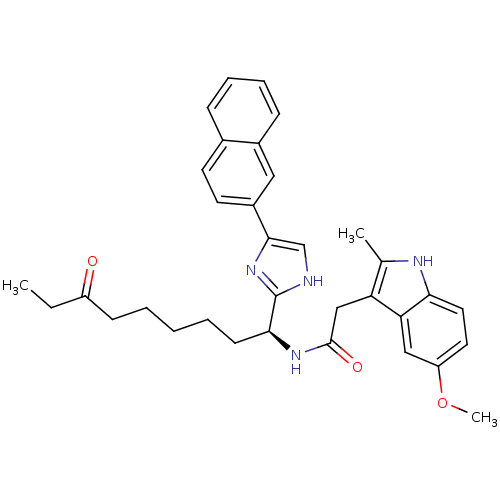
((S)-2-(5-methoxy-2-methyl-1H-indol-3-yl)-N-(1-(5-(...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)Cc1c(C)[nH]c2ccc(OC)cc12)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C34H38N4O3/c1-4-26(39)12-6-5-7-13-31(34-35-21-32(38-34)25-15-14-23-10-8-9-11-24(23)18-25)37-33(40)20-28-22(2)36-30-17-16-27(41-3)19-29(28)30/h8-11,14-19,21,31,36H,4-7,12-13,20H2,1-3H3,(H,35,38)(H,37,40)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50547569
(CHEMBL4764193)Show SMILES [H][C@@]12C[C@H](F)CN1C(=O)[C@H](CC(O)=O)NC(=O)[C@]([H])(CCC(=O)NC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@H](CCC(O)=O)NC2=O)[C@@H](C)O)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCCNC(N)=N |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115738
BindingDB Entry DOI: 10.7270/Q2QV3R4S |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50612445

(CHEMBL5282110) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50608555
(CHEMBL5269165) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50612445

(CHEMBL5282110) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50608555

(CHEMBL5269165) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50608555

(CHEMBL5269165) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50547572
(CHEMBL4788971)Show SMILES [H][C@@]12C[C@H](F)CN1C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCn1cc(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@H](CCC(O)=O)NC2=O)[C@@H](C)O)C(N)=O)nn1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@@H](N)CC(C)C)C(C)C |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115738
BindingDB Entry DOI: 10.7270/Q2QV3R4S |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50461256

(CHEMBL4228143)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(CN2CCN(C)CC2)cc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N |r| Show InChI InChI=1S/C25H37N5O4S/c1-19(2)16-22(24(32)28-25(18-26)8-14-35(33,34)15-9-25)27-23(31)21-6-4-20(5-7-21)17-30-12-10-29(3)11-13-30/h4-7,19,22H,8-17H2,1-3H3,(H,27,31)(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50547573
(CHEMBL4747827)Show SMILES [H][C@@]12C[C@H](F)CN1C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCn1cc(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@H](CCC(O)=O)NC2=O)[C@@H](C)O)C(N)=O)nn1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N)[C@@H](C)CC)[C@@H](C)CC |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115738
BindingDB Entry DOI: 10.7270/Q2QV3R4S |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50547571
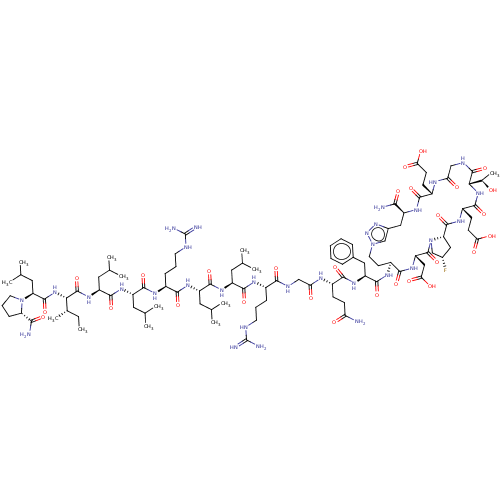
(CHEMBL4755277)Show SMILES [H][C@@]12C[C@H](F)CN1C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCn1cc(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@H](CCC(O)=O)NC2=O)[C@@H](C)O)C(N)=O)nn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)N1CCC[C@H]1C(N)=O)[C@@H](C)CC |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115738
BindingDB Entry DOI: 10.7270/Q2QV3R4S |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50547566

(CHEMBL4790062)Show SMILES [H][C@@]12C[C@H](F)CN1C(=O)[C@H](CC(O)=O)NC(=O)[C@]([H])(CCC(=O)NC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@H](CCC(O)=O)NC2=O)[C@@H](C)O)C(N)=O)NC(=O)CNC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@@H](N)CC(C)C)C(C)C |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115738
BindingDB Entry DOI: 10.7270/Q2QV3R4S |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50547567

(CHEMBL4781886)Show SMILES [H][C@@]12C[C@H](F)CN1C(=O)[C@H](CC(O)=O)NC(=O)[C@]([H])(CCC(=O)NC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@H](CCC(O)=O)NC2=O)[C@@H](C)O)C(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N)[C@@H](C)CC)[C@@H](C)CC |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115738
BindingDB Entry DOI: 10.7270/Q2QV3R4S |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50352268
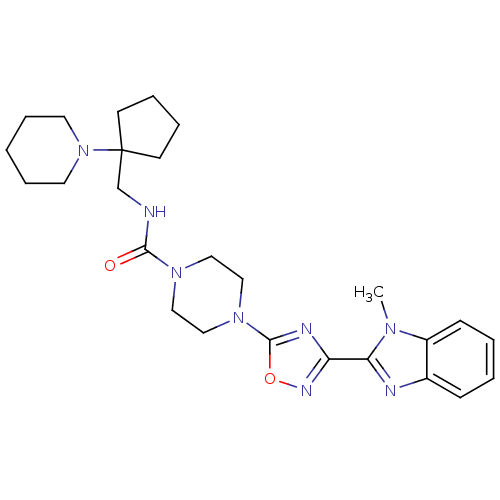
(CHEMBL1822467)Show SMILES Cn1c(nc2ccccc12)-c1noc(n1)N1CCN(CC1)C(=O)NCC1(CCCC1)N1CCCCC1 Show InChI InChI=1S/C26H36N8O2/c1-31-21-10-4-3-9-20(21)28-23(31)22-29-25(36-30-22)33-17-15-32(16-18-33)24(35)27-19-26(11-5-6-12-26)34-13-7-2-8-14-34/h3-4,9-10H,2,5-8,11-19H2,1H3,(H,27,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 2% fetal calf serum |
Bioorg Med Chem Lett 21: 5283-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.030
BindingDB Entry DOI: 10.7270/Q24F1R35 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50592392
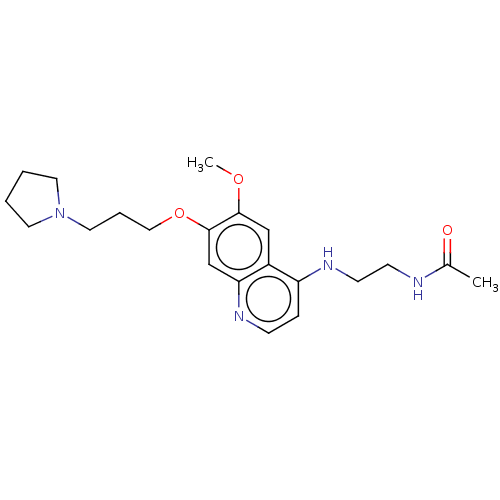
(CHEMBL5193402) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50592390

(CHEMBL5172085) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50446376

(CHEMBL3109630)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(N)C21CCC1 |t:19| Show InChI InChI=1S/C19H27N3O2/c1-23-16-12-14-15(21-18(20)19(14)6-4-7-19)13-17(16)24-11-5-10-22-8-2-3-9-22/h12-13H,2-11H2,1H3,(H2,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50352268

(CHEMBL1822467)Show SMILES Cn1c(nc2ccccc12)-c1noc(n1)N1CCN(CC1)C(=O)NCC1(CCCC1)N1CCCCC1 Show InChI InChI=1S/C26H36N8O2/c1-31-21-10-4-3-9-20(21)28-23(31)22-29-25(36-30-22)33-17-15-32(16-18-33)24(35)27-19-26(11-5-6-12-26)34-13-7-2-8-14-34/h3-4,9-10H,2,5-8,11-19H2,1H3,(H,27,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Displacement of BODIPY-cyclopamine from Smo expressed in COS-1 cells after 4 to 6 hrs by Flow Cytometry analysis in presence of 20% normal human seru... |
Bioorg Med Chem Lett 21: 5283-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.030
BindingDB Entry DOI: 10.7270/Q24F1R35 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461267
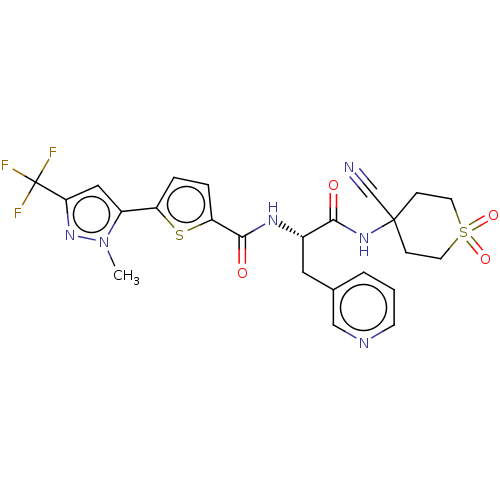
(CHEMBL4227427)Show SMILES Cn1nc(cc1-c1ccc(s1)C(=O)N[C@@H](Cc1cccnc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C24H23F3N6O4S2/c1-33-17(12-20(32-33)24(25,26)27)18-4-5-19(38-18)22(35)30-16(11-15-3-2-8-29-13-15)21(34)31-23(14-28)6-9-39(36,37)10-7-23/h2-5,8,12-13,16H,6-7,9-11H2,1H3,(H,30,35)(H,31,34)/t16-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50352419
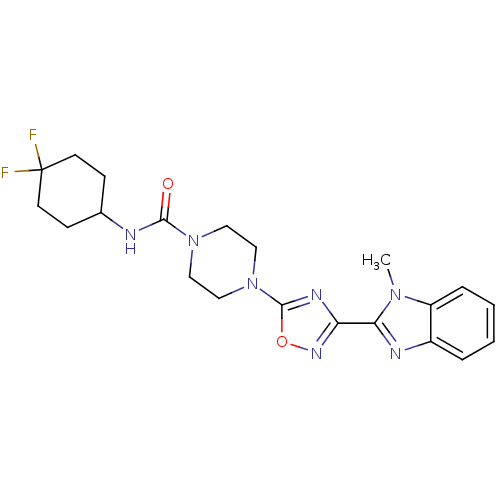
(CHEMBL1823864)Show SMILES Cn1c(nc2ccccc12)-c1noc(n1)N1CCN(CC1)C(=O)NC1CCC(F)(F)CC1 Show InChI InChI=1S/C21H25F2N7O2/c1-28-16-5-3-2-4-15(16)25-18(28)17-26-20(32-27-17)30-12-10-29(11-13-30)19(31)24-14-6-8-21(22,23)9-7-14/h2-5,14H,6-13H2,1H3,(H,24,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Displacement of Bodipy-labelled cyclopamine from Smo expressed in COS-1 cells in presence of 2% FBS after 4 to 6 hrs by FACS flow cytometric analysis |
Bioorg Med Chem Lett 21: 5274-82 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.031
BindingDB Entry DOI: 10.7270/Q2MC90DS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data