

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATP-dependent 6-phosphofructokinase (Trypanosoma brucei) | BDBM50446088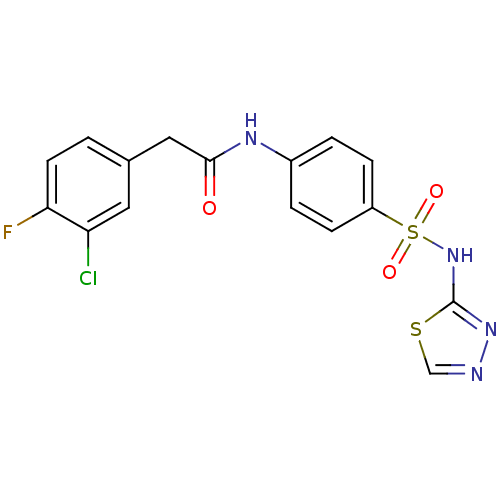 (CHEMBL3108870) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Competitive inhibition Trypanosoma brucei PFK using fructose-6-phosphate as substrate by Line-weaver Burk plot analysis | ACS Med Chem Lett 5: 12-7 (2014) Article DOI: 10.1021/ml400259d BindingDB Entry DOI: 10.7270/Q2X34ZX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent 6-phosphofructokinase (Trypanosoma brucei) | BDBM50446088 (CHEMBL3108870) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Mixed type inhibition Trypanosoma brucei PFK using ATP as substrate by Line-weaver Burk plot analysis | ACS Med Chem Lett 5: 12-7 (2014) Article DOI: 10.1021/ml400259d BindingDB Entry DOI: 10.7270/Q2X34ZX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443390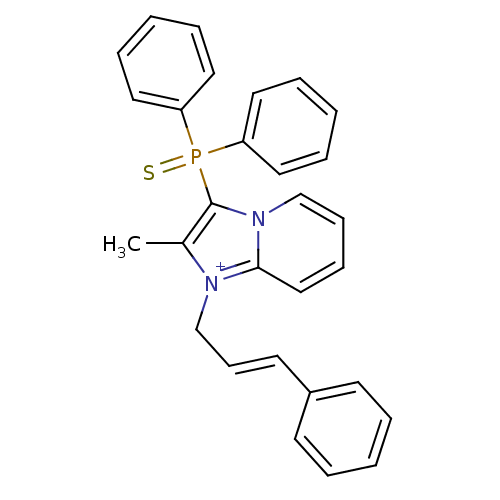 (CHEMBL1474387) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced calcium mobilization aft... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546980 (CHEMBL4792513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322839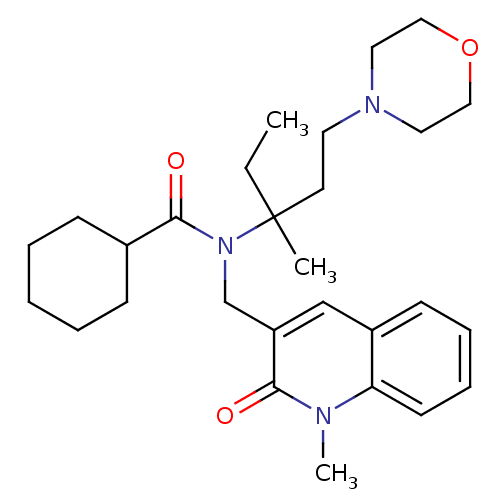 (CHEMBL1210313 | N-(3-methyl-1-morpholinopentan-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as cAMP level after 30 mins by phosphate-buffered sal... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322839 (CHEMBL1210313 | N-(3-methyl-1-morpholinopentan-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Displacement of [125I]Tyr10-NPS from neuropeptide S receptor (unknown origin) | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443390 (CHEMBL1474387) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced ERK activation after 20 ... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443406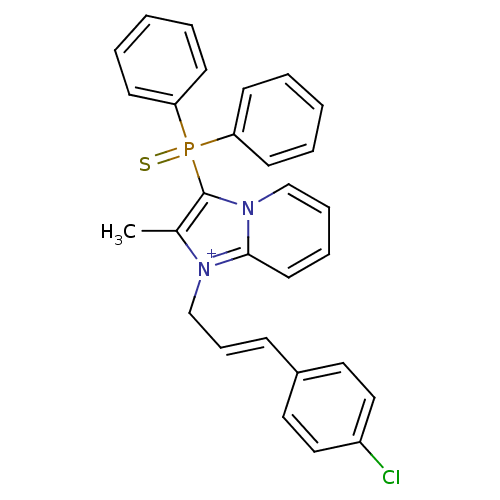 (CHEMBL3086823) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced calcium mobilization aft... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546969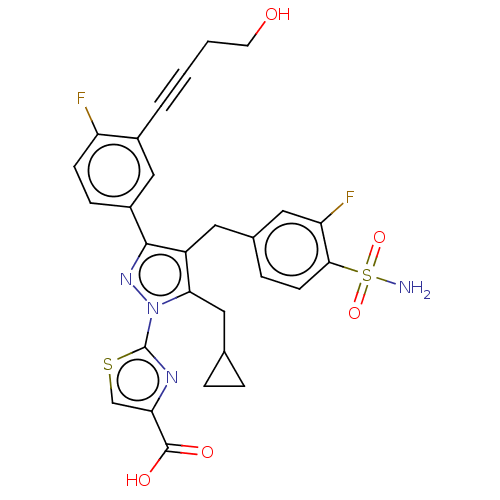 (CHEMBL4786682 | US11247971, Cmpd ID 409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443409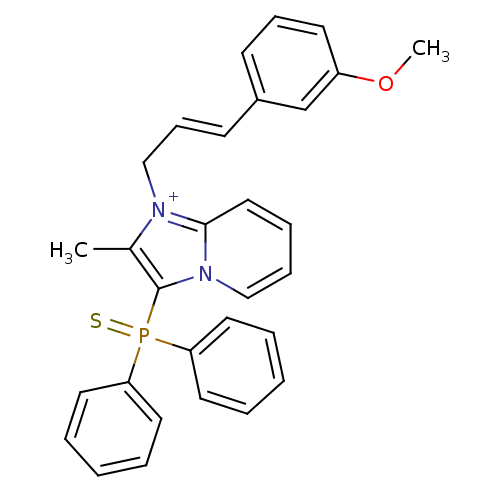 (CHEMBL3086836) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced calcium mobilization aft... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443400 (CHEMBL3086821) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced calcium mobilization aft... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443408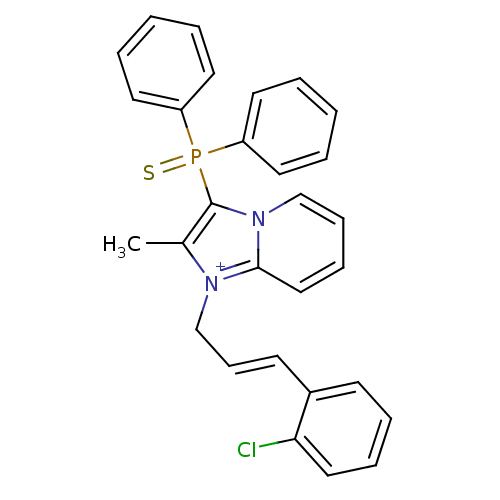 (CHEMBL3086837) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced calcium mobilization aft... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443390 (CHEMBL1474387) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Displacement of [125I]Tyr10-NPS from human neuropeptide S receptor expressed in CHO cells after 1.5 hrs by liquid scintillation counting | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443405 (CHEMBL3086824) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced calcium mobilization aft... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546981 (CHEMBL4797357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489091 (2-(5- (cyclopropylmethyl)- 3-(4-fluoro-3- ((tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489090 (2-(5- (cyclopropylmethyl)- 3-(4-fluoro-3- ((tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM537077 (US11247971, Cmpd ID 400) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443391 (CHEMBL469695) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as cAMP level after 30 mins by phosphate-buffered sal... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443404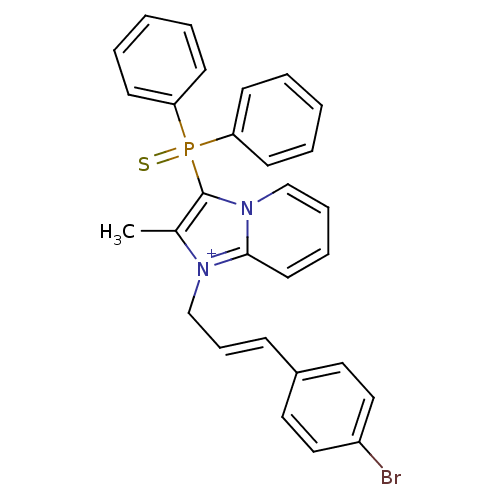 (CHEMBL3086825) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced calcium mobilization aft... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546978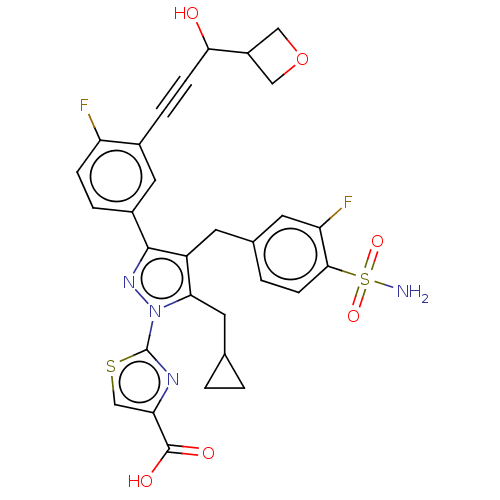 (CHEMBL4752940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489160 (2-(3-(3-(tert- butylcarbamoyl)-4- fluorophenyl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443392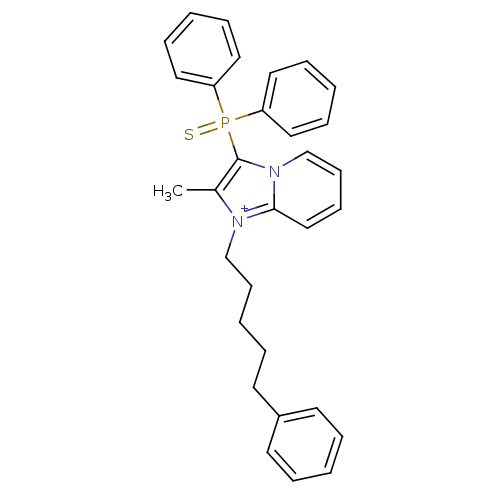 (CHEMBL1472563) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced ERK activation after 20 ... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322839 (CHEMBL1210313 | N-(3-methyl-1-morpholinopentan-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Displacement of [125I]Tyr10-NPS from human neuropeptide S receptor expressed in CHO cells after 1.5 hrs by liquid scintillation counting | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546975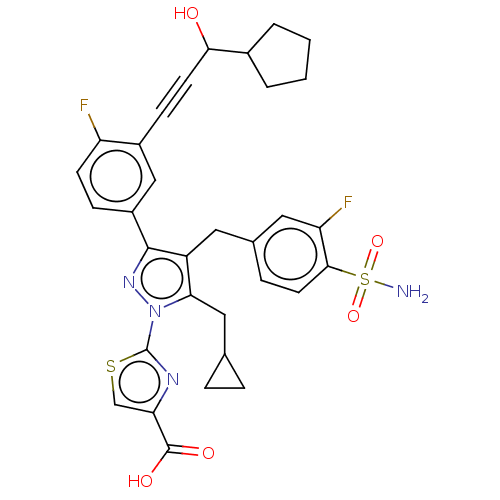 (CHEMBL4749903) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50569440 (CHEMBL4877988 | US11752138, Compound 152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546979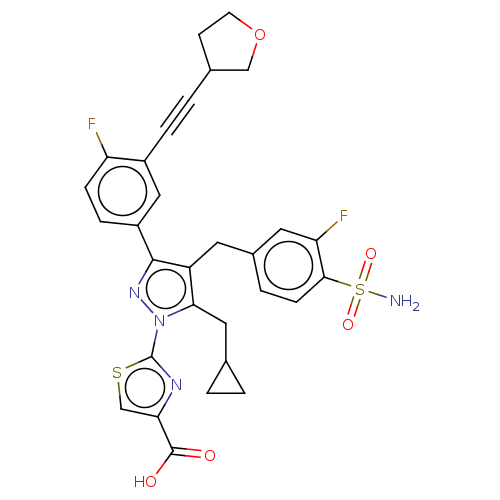 (CHEMBL4747300 | US11247971, Cmpd ID 423) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546977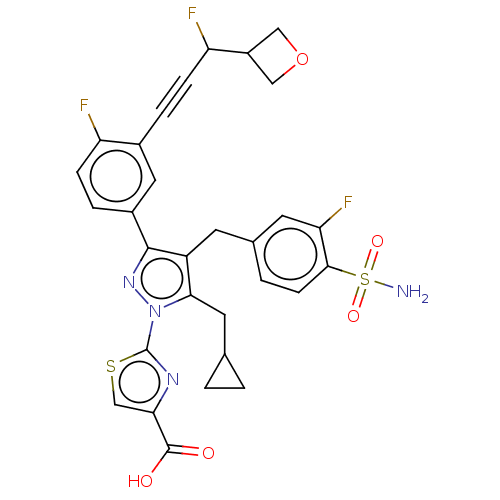 (CHEMBL4759378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM50250655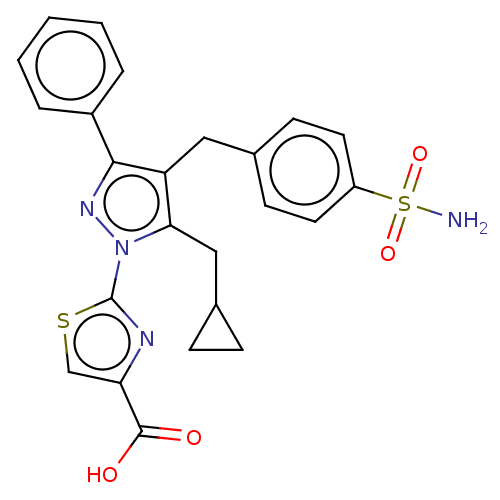 (CHEMBL4059985 | US10961200, Compound 189 | US11247...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human erythrocytes LDHB using sodium pyruvate as substrate after 5 mins in presence of NAPDH by diaphorase/resazurin based fluorescence... | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546998 (CHEMBL4790159 | US11247971, Cmpd ID 405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546970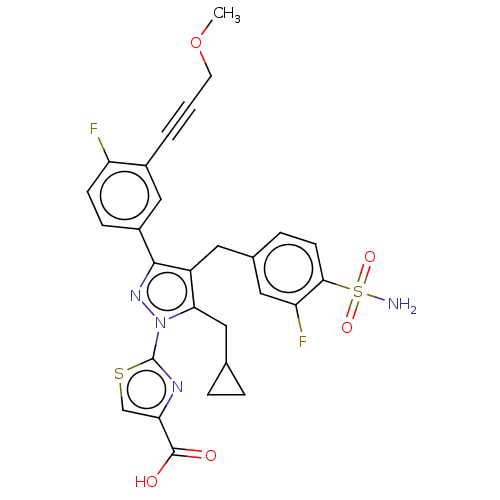 (CHEMBL4783945 | US11247971, Cmpd ID 404) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489161 (2-(3-(3- (benzylcarbamoyl)-4- fluorophenyl)-5- (cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM536955 (US11247971, Cmpd ID 278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489092 (2-(3-(3- cydopropoxy-4- fluorophenyl)-5- (cyclopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546989 (CHEMBL4759499 | US11247971, Cmpd ID 417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50250655 (CHEMBL4059985 | US10961200, Compound 189 | US11247...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human liver LDHA using sodium pyruvate as substrate after 5 mins in presence of NAPDH and in absence of EDTA by diaphorase/resazurin ba... | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546971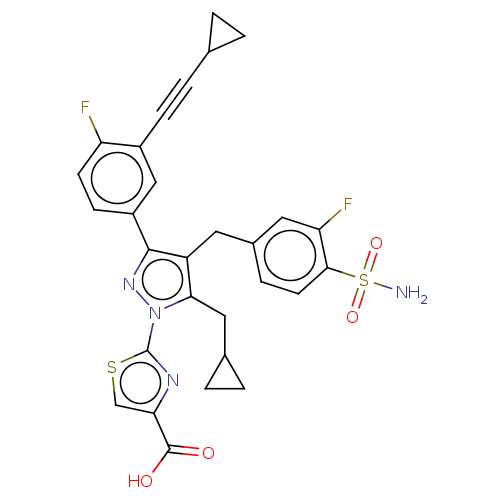 (CHEMBL4777867 | US11247971, Cmpd ID 262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443399 (CHEMBL3086822) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced calcium mobilization aft... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546999 (CHEMBL4786717) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50547000 (CHEMBL4783252 | US11247971, Cmpd ID 270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546990 (CHEMBL4794789) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50250655 (CHEMBL4059985 | US10961200, Compound 189 | US11247...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Binding affinity to human His-tagged LDHA in presence of NADH by SPR assay | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546963 (CHEMBL4760911 | US11247971, Cmpd ID 410) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443398 (CHEMBL1514777) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced ERK activation after 20 ... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50443407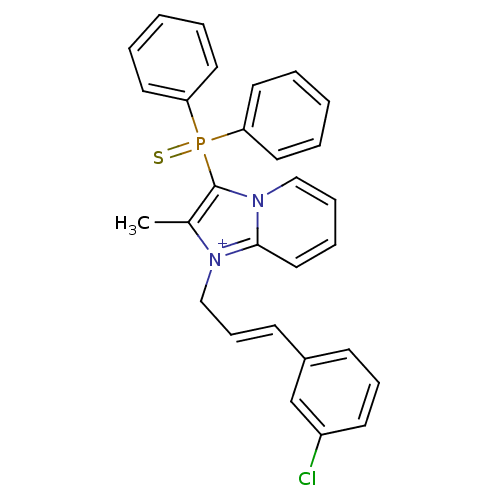 (CHEMBL3086838) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced calcium mobilization aft... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM197160 (GNE-140 (6)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of human LDHA using sodium pyruvate as substrate in presence of NAPDH | J Med Chem 60: 9184-9204 (2017) Article DOI: 10.1021/acs.jmedchem.7b00941 BindingDB Entry DOI: 10.7270/Q2DJ5J28 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50546976 (CHEMBL4751495) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00916 BindingDB Entry DOI: 10.7270/Q2057KJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322839 (CHEMBL1210313 | N-(3-methyl-1-morpholinopentan-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) assessed as intracellular calcium level by cell based assay | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50331943 (5-phenyl-2-(2-(piperidine-1-carbonyl)phenyl)-2,3-d...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) assessed as intracellular calcium level by cell based assay | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide S receptor (Homo sapiens (Human)) | BDBM50322839 (CHEMBL1210313 | N-(3-methyl-1-morpholinopentan-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced ERK activation after 20 ... | J Med Chem 56: 9045-56 (2013) Article DOI: 10.1021/jm400904m BindingDB Entry DOI: 10.7270/Q2NG4S3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2204 total ) | Next | Last >> |