 Found 60 hits with Last Name = 'bum-erdene' and Initial = 'k'
Found 60 hits with Last Name = 'bum-erdene' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549787
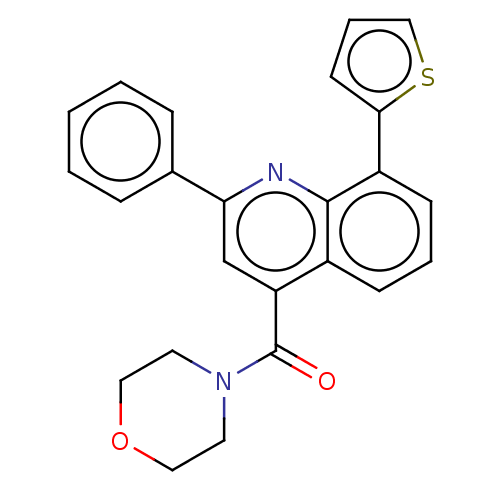
(CHEMBL4791246)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)-c1cccs1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549794
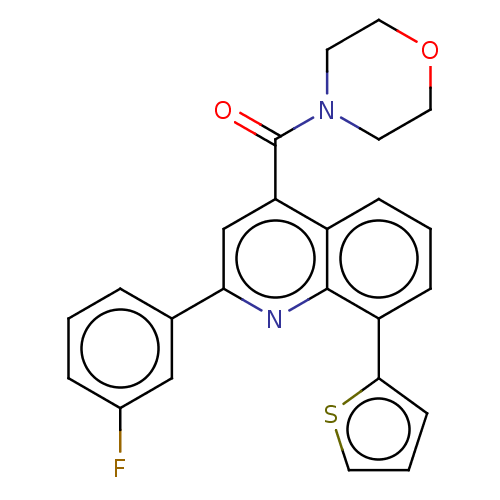
(CHEMBL4753144)Show SMILES Fc1cccc(c1)-c1cc(C(=O)N2CCOCC2)c2cccc(-c3cccs3)c2n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549790

(CHEMBL4748577)Show SMILES COc1cccc(c1)-c1cc(C(=O)N2CCOCC2)c2cccc(-c3cccs3)c2n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549789
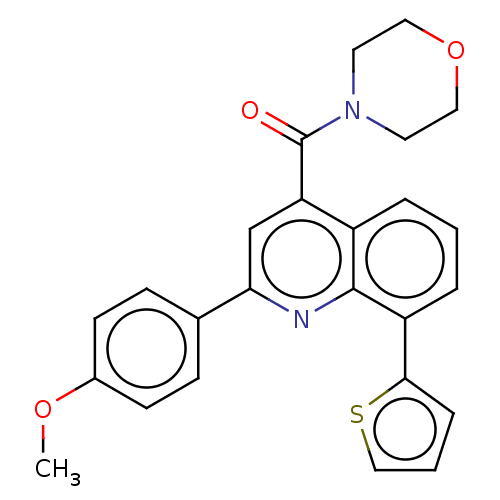
(CHEMBL4752534)Show SMILES COc1ccc(cc1)-c1cc(C(=O)N2CCOCC2)c2cccc(-c3cccs3)c2n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549787

(CHEMBL4791246)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)-c1cccs1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549788

(CHEMBL4748144)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)-c1cncs1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549801
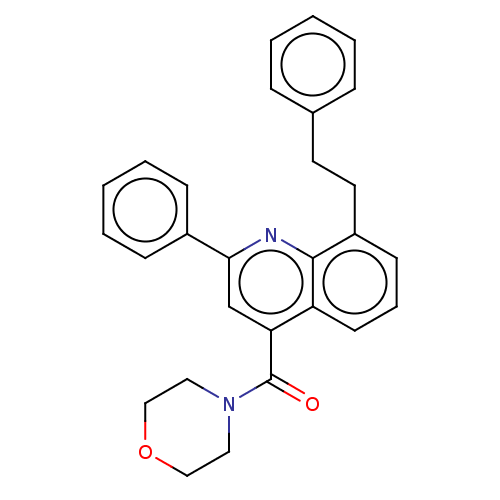
(CHEMBL4742729)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(CCc3ccccc3)cccc12)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549794

(CHEMBL4753144)Show SMILES Fc1cccc(c1)-c1cc(C(=O)N2CCOCC2)c2cccc(-c3cccs3)c2n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549796
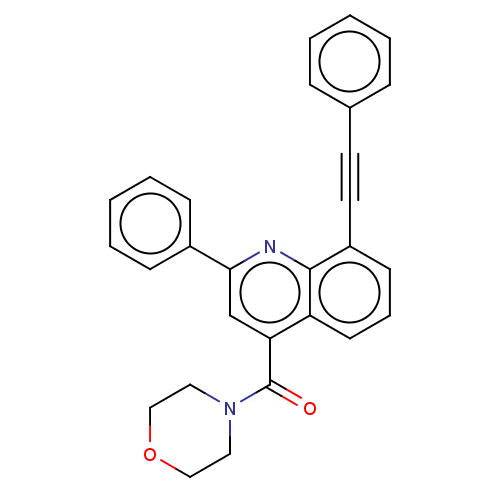
(CHEMBL4740414)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)C#Cc1ccccc1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549793

(CHEMBL4760716)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)-c1cccs1)-c1cccnc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549787

(CHEMBL4791246)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)-c1cccs1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549792

(CHEMBL4790155)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cncc12)-c1cccs1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549800
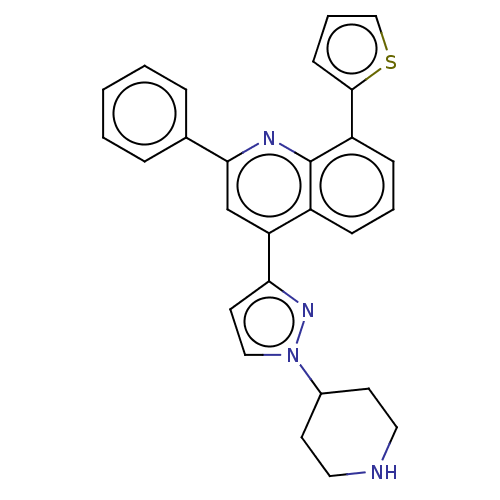
(CHEMBL4748584)Show SMILES C1CC(CCN1)n1ccc(n1)-c1cc(nc2c(cccc12)-c1cccs1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549797

(CHEMBL4757892)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)-c1ccccc1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549792

(CHEMBL4790155)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cncc12)-c1cccs1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549790

(CHEMBL4748577)Show SMILES COc1cccc(c1)-c1cc(C(=O)N2CCOCC2)c2cccc(-c3cccs3)c2n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549789

(CHEMBL4752534)Show SMILES COc1ccc(cc1)-c1cc(C(=O)N2CCOCC2)c2cccc(-c3cccs3)c2n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549799
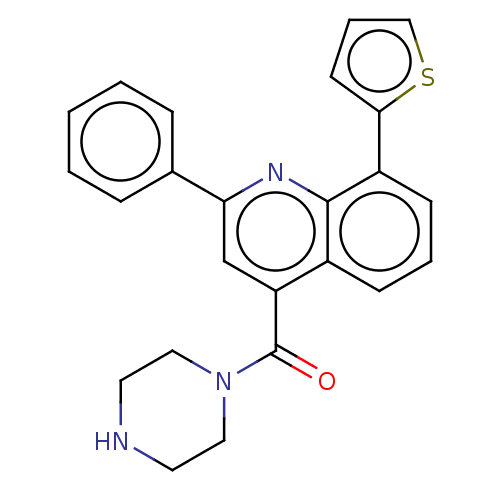
(CHEMBL4740458)Show SMILES O=C(N1CCNCC1)c1cc(nc2c(cccc12)-c1cccs1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549791

(CHEMBL4755034)Show SMILES Clc1cccc(c1)N1CCN(CC1)C(=O)c1cc(nc2c(cccc12)-c1cccs1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549797

(CHEMBL4757892)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)-c1ccccc1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549793

(CHEMBL4760716)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)-c1cccs1)-c1cccnc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549795
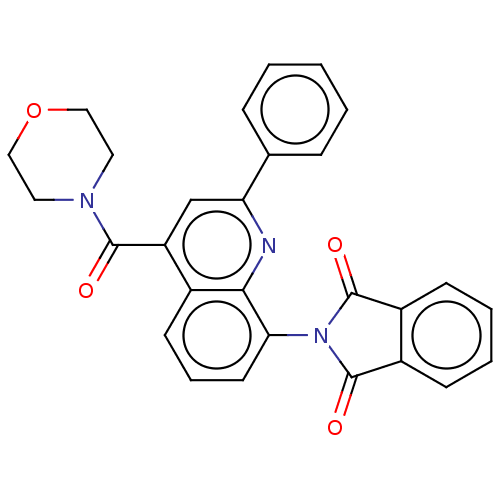
(CHEMBL4748990)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)N1C(=O)c2ccccc2C1=O)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549796

(CHEMBL4740414)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)C#Cc1ccccc1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549795

(CHEMBL4748990)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)N1C(=O)c2ccccc2C1=O)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549788

(CHEMBL4748144)Show SMILES O=C(N1CCOCC1)c1cc(nc2c(cccc12)-c1cncs1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549798
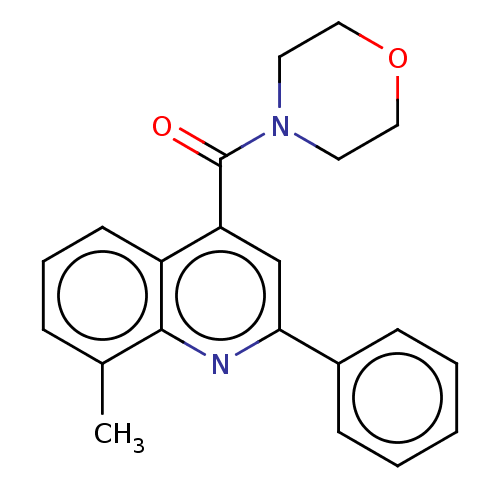
(CHEMBL4748026) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AE147-FAM peptide binding to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 ce... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50549798

(CHEMBL4748026) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant soluble form urokinase-type plasminogen activator receptor (unknown origin) expressed in S2 cells assessed as inhibit... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00422
BindingDB Entry DOI: 10.7270/Q21C21G5 |
More data for this
Ligand-Target Pair | |
Galectin-8
(Homo sapiens (Human)) | BDBM50605164

(CHEMBL5202629)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1cccc(c1)[N+]([O-])=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.00E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-1
(Homo sapiens (Human)) | BDBM50077225
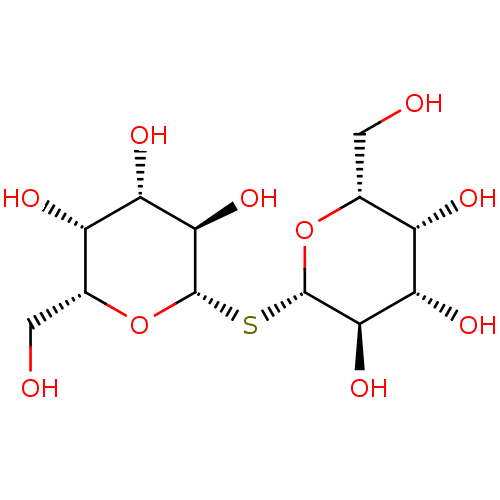
((2R,3R,4S,5R,6S)-2-(hydroxymethyl)-6-{[(2S,3R,4S,5...)Show SMILES OC[C@H]1O[C@@H](S[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H22O10S/c13-1-3-5(15)7(17)9(19)11(21-3)23-12-10(20)8(18)6(16)4(2-14)22-12/h3-20H,1-2H2/t3-,4-,5+,6+,7+,8+,9-,10-,11+,12+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Galectin-3
(Homo sapiens (Human)) | BDBM50077225

((2R,3R,4S,5R,6S)-2-(hydroxymethyl)-6-{[(2S,3R,4S,5...)Show SMILES OC[C@H]1O[C@@H](S[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H22O10S/c13-1-3-5(15)7(17)9(19)11(21-3)23-12-10(20)8(18)6(16)4(2-14)22-12/h3-20H,1-2H2/t3-,4-,5+,6+,7+,8+,9-,10-,11+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Galectin-8
(Homo sapiens (Human)) | BDBM50077225

((2R,3R,4S,5R,6S)-2-(hydroxymethyl)-6-{[(2S,3R,4S,5...)Show SMILES OC[C@H]1O[C@@H](S[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H22O10S/c13-1-3-5(15)7(17)9(19)11(21-3)23-12-10(20)8(18)6(16)4(2-14)22-12/h3-20H,1-2H2/t3-,4-,5+,6+,7+,8+,9-,10-,11+,12+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-4
(Homo sapiens (Human)) | BDBM50077225

((2R,3R,4S,5R,6S)-2-(hydroxymethyl)-6-{[(2S,3R,4S,5...)Show SMILES OC[C@H]1O[C@@H](S[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H22O10S/c13-1-3-5(15)7(17)9(19)11(21-3)23-12-10(20)8(18)6(16)4(2-14)22-12/h3-20H,1-2H2/t3-,4-,5+,6+,7+,8+,9-,10-,11+,12+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 4.10E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-8
(Homo sapiens (Human)) | BDBM50605163

(CHEMBL5193025)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccc(cc1[N+]([O-])=O)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.90E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-8
(Homo sapiens (Human)) | BDBM50605162

(CHEMBL5189715)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccc(F)cc1[N+]([O-])=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 9.00E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-8
(Homo sapiens (Human)) | BDBM50605161

(CHEMBL5195652)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccc(Cl)cc1[N+]([O-])=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.50E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-8
(Homo sapiens (Human)) | BDBM50605161

(CHEMBL5195652)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccc(Cl)cc1[N+]([O-])=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.00E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-4
(Homo sapiens (Human)) | BDBM50243731
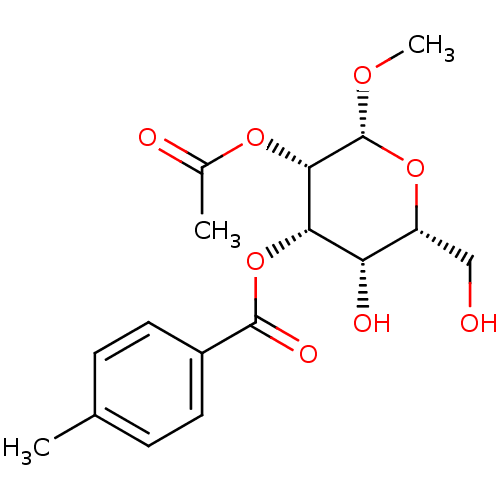
((2R,3S,4S,5S,6R)-3-acetoxy-5-hydroxy-6-(hydroxymet...)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C17H22O8/c1-9-4-6-11(7-5-9)16(21)25-14-13(20)12(8-18)24-17(22-3)15(14)23-10(2)19/h4-7,12-15,17-18,20H,8H2,1-3H3/t12-,13+,14+,15+,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.90E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-4
(Homo sapiens (Human)) | BDBM50605164

(CHEMBL5202629)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1cccc(c1)[N+]([O-])=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6.30E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-4
(Homo sapiens (Human)) | BDBM50605163

(CHEMBL5193025)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccc(cc1[N+]([O-])=O)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-4
(Homo sapiens (Human)) | BDBM50605162

(CHEMBL5189715)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccc(F)cc1[N+]([O-])=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-4
(Homo sapiens (Human)) | BDBM50605161

(CHEMBL5195652)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccc(Cl)cc1[N+]([O-])=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-4
(Homo sapiens (Human)) | BDBM50605165
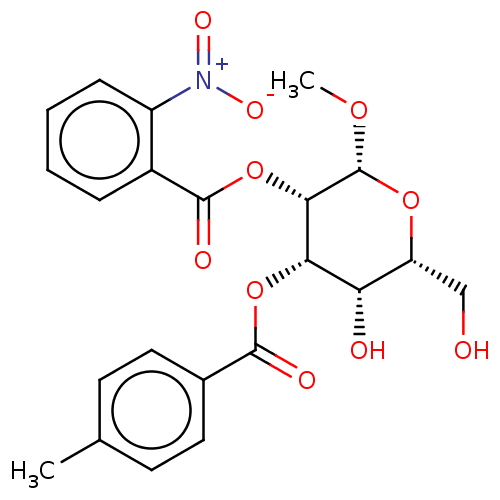
(CHEMBL5175908)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccccc1[N+]([O-])=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-4
(Homo sapiens (Human)) | BDBM50605164

(CHEMBL5202629)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1cccc(c1)[N+]([O-])=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.40E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-4
(Homo sapiens (Human)) | BDBM50605163

(CHEMBL5193025)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccc(cc1[N+]([O-])=O)C(F)(F)F |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.40E+6 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-4
(Homo sapiens (Human)) | BDBM50605162

(CHEMBL5189715)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccc(F)cc1[N+]([O-])=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 7.10E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-4
(Homo sapiens (Human)) | BDBM50605161

(CHEMBL5195652)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccc(Cl)cc1[N+]([O-])=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-4
(Homo sapiens (Human)) | BDBM50605165

(CHEMBL5175908)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccccc1[N+]([O-])=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.40E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | |
Galectin-3
(Homo sapiens (Human)) | BDBM50243731

((2R,3S,4S,5S,6R)-3-acetoxy-5-hydroxy-6-(hydroxymet...)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C17H22O8/c1-9-4-6-11(7-5-9)16(21)25-14-13(20)12(8-18)24-17(22-3)15(14)23-10(2)19/h4-7,12-15,17-18,20H,8H2,1-3H3/t12-,13+,14+,15+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 4.20E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Galectin-3
(Homo sapiens (Human)) | BDBM50605164

(CHEMBL5202629)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1cccc(c1)[N+]([O-])=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Galectin-3
(Homo sapiens (Human)) | BDBM50605163

(CHEMBL5193025)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OC(=O)c2ccc(C)cc2)[C@@H]1OC(=O)c1ccc(cc1[N+]([O-])=O)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01296
BindingDB Entry DOI: 10.7270/Q2FX7FKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data