 Found 152 hits with Last Name = 'burger' and Initial = 'd'
Found 152 hits with Last Name = 'burger' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594427

(CHEMBL5172496)Show SMILES OC(=O)CCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:16:17:20:24.23.22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50327809
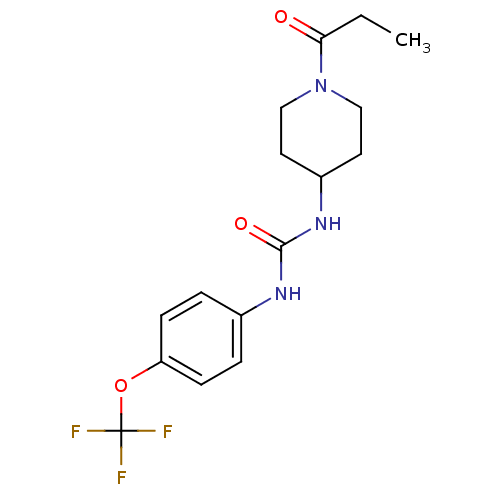
(1-(1-Propionylpiperidin-4-yl)-3-(4-(trifluorometho...)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H20F3N3O3/c1-2-14(23)22-9-7-12(8-10-22)21-15(24)20-11-3-5-13(6-4-11)25-16(17,18)19/h3-6,12H,2,7-10H2,1H3,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594426
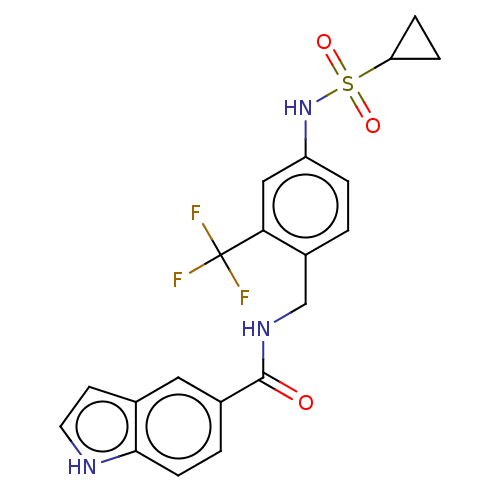
(CHEMBL5177249)Show SMILES FC(F)(F)c1cc(NS(=O)(=O)C2CC2)ccc1CNC(=O)c1ccc2[nH]ccc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50209759
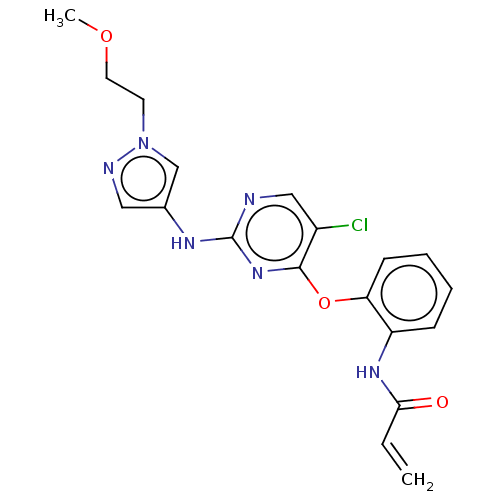
(CHEMBL3884839)Show SMILES COCCn1cc(Nc2ncc(Cl)c(Oc3ccccc3NC(=O)C=C)n2)cn1 Show InChI InChI=1S/C19H19ClN6O3/c1-3-17(27)24-15-6-4-5-7-16(15)29-18-14(20)11-21-19(25-18)23-13-10-22-26(12-13)8-9-28-2/h3-7,10-12H,1,8-9H2,2H3,(H,24,27)(H,21,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Inc
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) |
Bioorg Med Chem 25: 1320-1328 (2017)
Article DOI: 10.1016/j.bmc.2016.11.034
BindingDB Entry DOI: 10.7270/Q2JH3PNP |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50209748
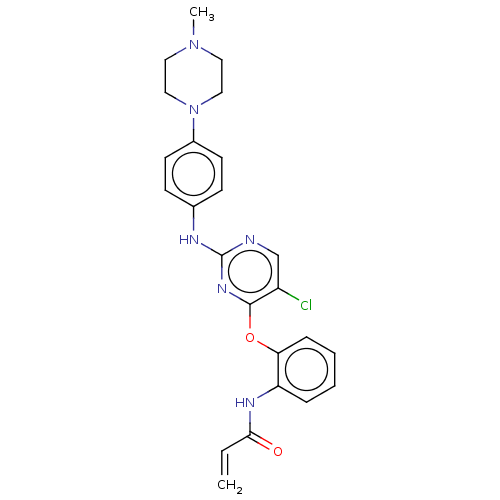
(CHEMBL3884960)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(Oc3ccccc3NC(=O)C=C)n2)cc1 Show InChI InChI=1S/C24H25ClN6O2/c1-3-22(32)28-20-6-4-5-7-21(20)33-23-19(25)16-26-24(29-23)27-17-8-10-18(11-9-17)31-14-12-30(2)13-15-31/h3-11,16H,1,12-15H2,2H3,(H,28,32)(H,26,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Inc
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) |
Bioorg Med Chem 25: 1320-1328 (2017)
Article DOI: 10.1016/j.bmc.2016.11.034
BindingDB Entry DOI: 10.7270/Q2JH3PNP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594428

(CHEMBL5192349)Show SMILES COc1ccc(CNC(=O)[C@@H]2CCC[C@@H](C2)Nc2ccc([N+]([O-])=O)c3nonc23)c(c1)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50209760
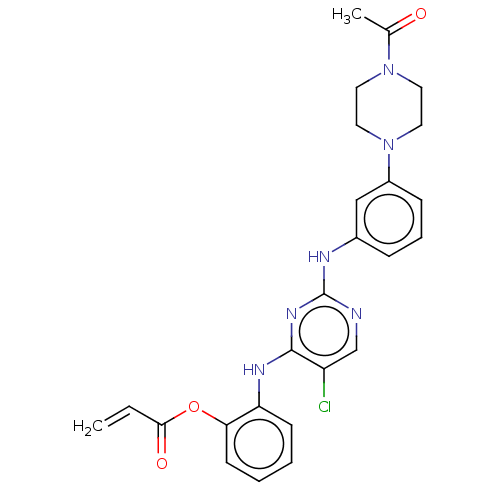
(CHEMBL3885421)Show SMILES CC(=O)N1CCN(CC1)c1cccc(Nc2ncc(Cl)c(Nc3ccccc3OC(=O)C=C)n2)c1 Show InChI InChI=1S/C25H25ClN6O3/c1-3-23(34)35-22-10-5-4-9-21(22)29-24-20(26)16-27-25(30-24)28-18-7-6-8-19(15-18)32-13-11-31(12-14-32)17(2)33/h3-10,15-16H,1,11-14H2,2H3,(H2,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Inc
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) by LanthaScreen assay |
Bioorg Med Chem 25: 1320-1328 (2017)
Article DOI: 10.1016/j.bmc.2016.11.034
BindingDB Entry DOI: 10.7270/Q2JH3PNP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593227
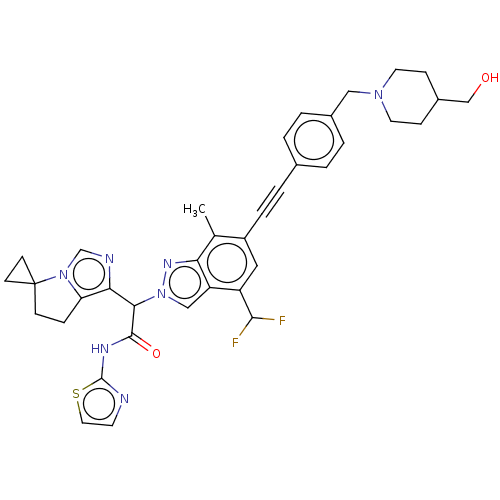
(CHEMBL5177023)Show SMILES Cc1c(cc(C(F)F)c2cn(nc12)C(C(=O)Nc1nccs1)c1ncn2c1CCC21CC1)C#Cc1ccc(CN2CCC(CO)CC2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50209759

(CHEMBL3884839)Show SMILES COCCn1cc(Nc2ncc(Cl)c(Oc3ccccc3NC(=O)C=C)n2)cn1 Show InChI InChI=1S/C19H19ClN6O3/c1-3-17(27)24-15-6-4-5-7-16(15)29-18-14(20)11-21-19(25-18)23-13-10-22-26(12-13)8-9-28-2/h3-7,10-12H,1,8-9H2,2H3,(H,24,27)(H,21,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Inc
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) by LanthaScreen assay |
Bioorg Med Chem 25: 1320-1328 (2017)
Article DOI: 10.1016/j.bmc.2016.11.034
BindingDB Entry DOI: 10.7270/Q2JH3PNP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594427

(CHEMBL5172496)Show SMILES OC(=O)CCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:16:17:20:24.23.22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593214
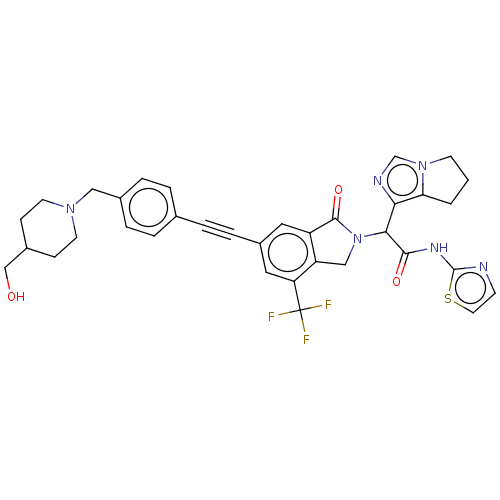
(CHEMBL5189574)Show SMILES OCC1CCN(Cc2ccc(cc2)C#Cc2cc3C(=O)N(Cc3c(c2)C(F)(F)F)C(C(=O)Nc2nccs2)c2ncn3CCCc23)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593226
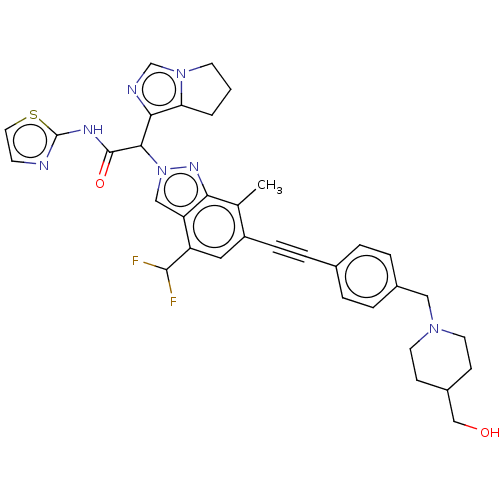
(CHEMBL5184760)Show SMILES Cc1c(cc(C(F)F)c2cn(nc12)C(C(=O)Nc1nccs1)c1ncn2CCCc12)C#Cc1ccc(CN2CCC(CO)CC2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED)
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in renal leiomyoblastoma cells |
J Med Chem 58: 8054-65 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00851
BindingDB Entry DOI: 10.7270/Q2K0763C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50209653
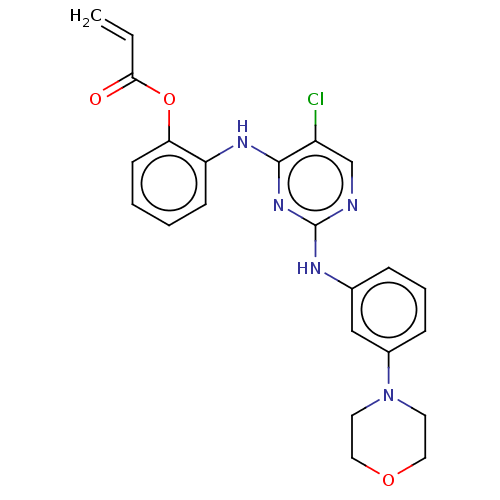
(CHEMBL3884616)Show SMILES Clc1cnc(Nc2cccc(c2)N2CCOCC2)nc1Nc1ccccc1OC(=O)C=C Show InChI InChI=1S/C23H22ClN5O3/c1-2-21(30)32-20-9-4-3-8-19(20)27-22-18(24)15-25-23(28-22)26-16-6-5-7-17(14-16)29-10-12-31-13-11-29/h2-9,14-15H,1,10-13H2,(H2,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Inc
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) by LanthaScreen assay |
Bioorg Med Chem 25: 1320-1328 (2017)
Article DOI: 10.1016/j.bmc.2016.11.034
BindingDB Entry DOI: 10.7270/Q2JH3PNP |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50209662
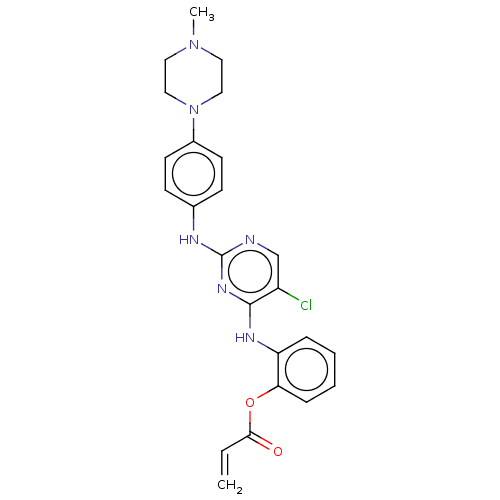
(CHEMBL3884569)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(Nc3ccccc3OC(=O)C=C)n2)cc1 Show InChI InChI=1S/C24H25ClN6O2/c1-3-22(32)33-21-7-5-4-6-20(21)28-23-19(25)16-26-24(29-23)27-17-8-10-18(11-9-17)31-14-12-30(2)13-15-31/h3-11,16H,1,12-15H2,2H3,(H2,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Inc
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) by LanthaScreen assay |
Bioorg Med Chem 25: 1320-1328 (2017)
Article DOI: 10.1016/j.bmc.2016.11.034
BindingDB Entry DOI: 10.7270/Q2JH3PNP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593215
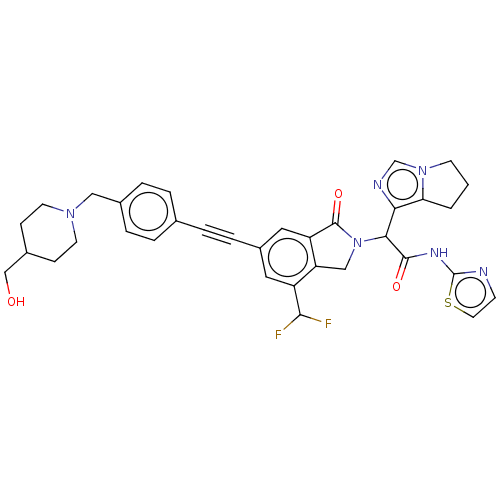
(CHEMBL5184539)Show SMILES OCC1CCN(Cc2ccc(cc2)C#Cc2cc3C(=O)N(Cc3c(c2)C(F)F)C(C(=O)Nc2nccs2)c2ncn3CCCc23)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593204

(CHEMBL5173979)Show SMILES Clc1cccc2C(=O)N(Cc12)C(C(=O)Nc1nccs1)c1ncn2CCCc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593219
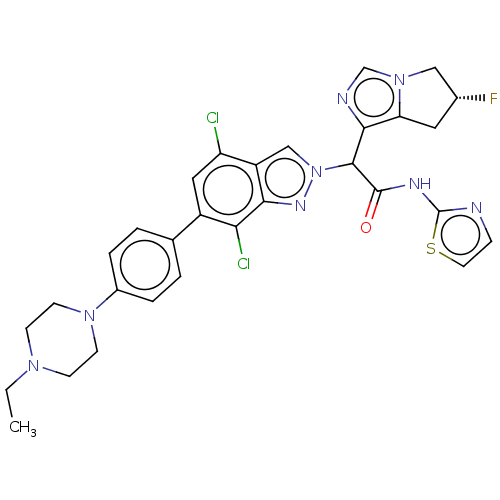
(CHEMBL5202945)Show SMILES CCN1CCN(CC1)c1ccc(cc1)-c1cc(Cl)c2cn(nc2c1Cl)C(C(=O)Nc1nccs1)c1ncn2C[C@H](F)Cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593216

(CHEMBL5183324)Show SMILES CC1(C)CCc2c(ncn12)C(N1Cc2c(cc(cc2C(F)F)C#Cc2ccc(CN3CCC(CO)CC3)cc2)C1=O)C(=O)Nc1nccs1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50209657
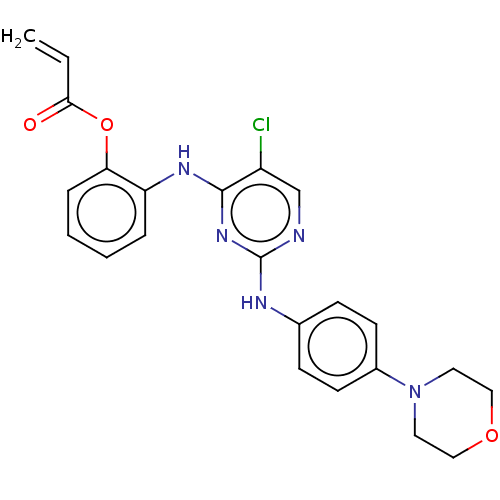
(CHEMBL3884229)Show SMILES Clc1cnc(Nc2ccc(cc2)N2CCOCC2)nc1Nc1ccccc1OC(=O)C=C Show InChI InChI=1S/C23H22ClN5O3/c1-2-21(30)32-20-6-4-3-5-19(20)27-22-18(24)15-25-23(28-22)26-16-7-9-17(10-8-16)29-11-13-31-14-12-29/h2-10,15H,1,11-14H2,(H2,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Inc
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) by LanthaScreen assay |
Bioorg Med Chem 25: 1320-1328 (2017)
Article DOI: 10.1016/j.bmc.2016.11.034
BindingDB Entry DOI: 10.7270/Q2JH3PNP |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50209660
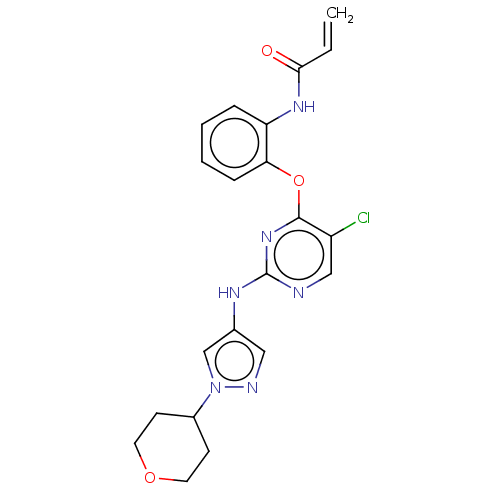
(CHEMBL3884281)Show SMILES Clc1cnc(Nc2cnn(c2)C2CCOCC2)nc1Oc1ccccc1NC(=O)C=C Show InChI InChI=1S/C21H21ClN6O3/c1-2-19(29)26-17-5-3-4-6-18(17)31-20-16(22)12-23-21(27-20)25-14-11-24-28(13-14)15-7-9-30-10-8-15/h2-6,11-13,15H,1,7-10H2,(H,26,29)(H,23,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Inc
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) by LanthaScreen assay |
Bioorg Med Chem 25: 1320-1328 (2017)
Article DOI: 10.1016/j.bmc.2016.11.034
BindingDB Entry DOI: 10.7270/Q2JH3PNP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593213
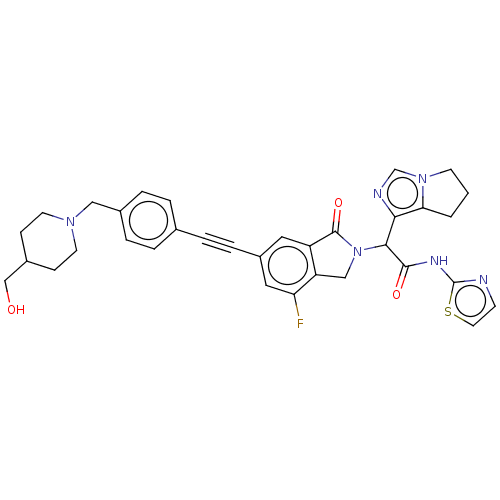
(CHEMBL5180839)Show SMILES OCC1CCN(Cc2ccc(cc2)C#Cc2cc3C(=O)N(Cc3c(F)c2)C(C(=O)Nc2nccs2)c2ncn3CCCc23)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593212

(CHEMBL5207479)Show SMILES OC1CCN(Cc2ccc(cc2)C#Cc2cc3C(=O)N(Cc3c(F)c2)C(C(=O)Nc2nccs2)c2ncn3CCCc23)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50209748

(CHEMBL3884960)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(Oc3ccccc3NC(=O)C=C)n2)cc1 Show InChI InChI=1S/C24H25ClN6O2/c1-3-22(32)28-20-6-4-5-7-21(20)33-23-19(25)16-26-24(29-23)27-17-8-10-18(11-9-17)31-14-12-30(2)13-15-31/h3-11,16H,1,12-15H2,2H3,(H,28,32)(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Inc
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) by LanthaScreen assay |
Bioorg Med Chem 25: 1320-1328 (2017)
Article DOI: 10.1016/j.bmc.2016.11.034
BindingDB Entry DOI: 10.7270/Q2JH3PNP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50042034
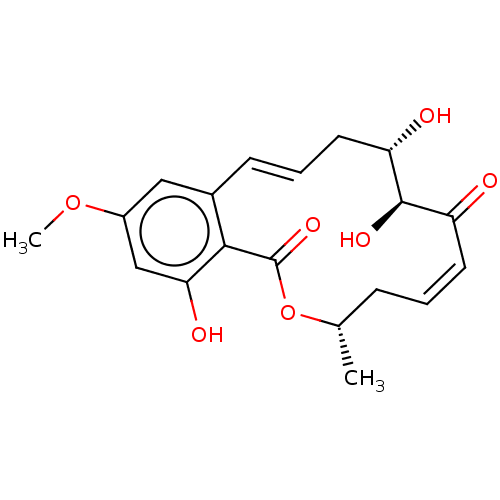
((5Z)-7-Oxozeaenol | 5Z-7-Oxozeaenol | CHEBI:67559 ...)Show SMILES COc1cc(O)c2c(c1)\C=C\C[C@H](O)[C@H](O)C(=O)\C=C/C[C@H](C)OC2=O |r,c:19,t:10| Show InChI InChI=1S/C19H22O7/c1-11-5-3-7-14(20)18(23)15(21)8-4-6-12-9-13(25-2)10-16(22)17(12)19(24)26-11/h3-4,6-7,9-11,15,18,21-23H,5,8H2,1-2H3/b6-4+,7-3-/t11-,15-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Inc
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) by LanthaScreen assay |
Bioorg Med Chem 25: 1320-1328 (2017)
Article DOI: 10.1016/j.bmc.2016.11.034
BindingDB Entry DOI: 10.7270/Q2JH3PNP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50453891
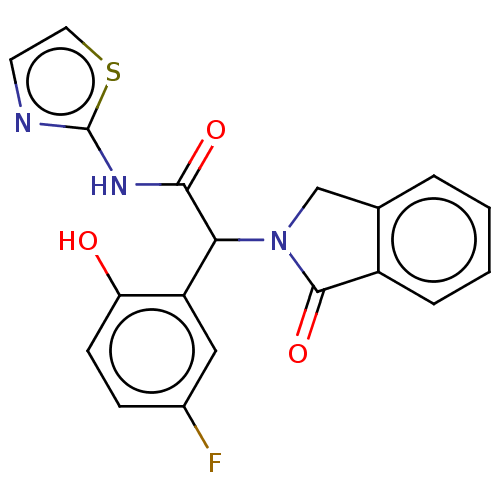
(CHEMBL4214567)Show SMILES Oc1ccc(F)cc1C(N1Cc2ccccc2C1=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H14FN3O3S/c20-12-5-6-15(24)14(9-12)16(17(25)22-19-21-7-8-27-19)23-10-11-3-1-2-4-13(11)18(23)26/h1-9,16,24H,10H2,(H,21,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593225

(CHEMBL5193772)Show SMILES OC1CCN(Cc2ccc(cc2)C#Cc2cc(F)c3cn(nc3c2)C(C(=O)Nc2nccs2)c2ncn3CCCc23)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED)
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in renal leiomyoblastoma cells |
J Med Chem 58: 8054-65 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00851
BindingDB Entry DOI: 10.7270/Q2K0763C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593222
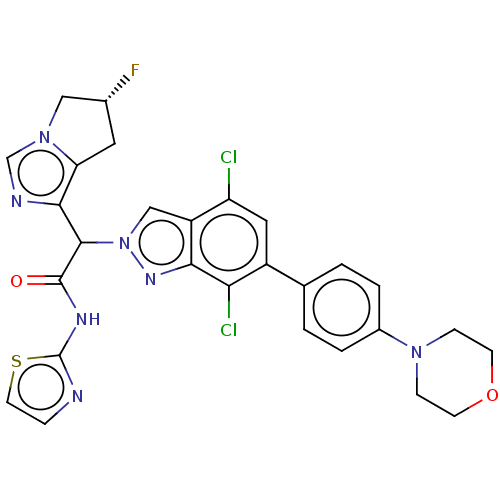
(CHEMBL5208056)Show SMILES F[C@@H]1Cc2c(ncn2C1)C(C(=O)Nc1nccs1)n1cc2c(Cl)cc(-c3ccc(cc3)N3CCOCC3)c(Cl)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593223
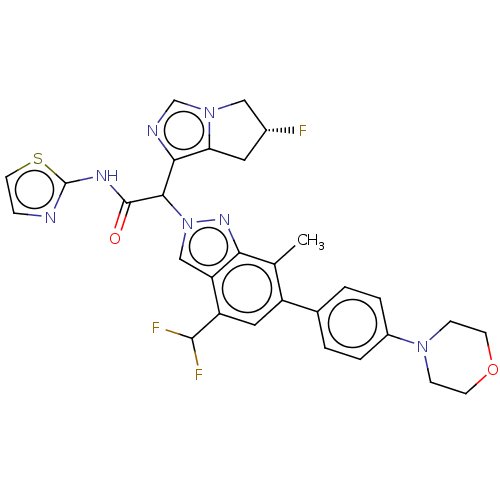
(CHEMBL5193594)Show SMILES Cc1c(cc(C(F)F)c2cn(nc12)C(C(=O)Nc1nccs1)c1ncn2C[C@H](F)Cc12)-c1ccc(cc1)N1CCOCC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593217
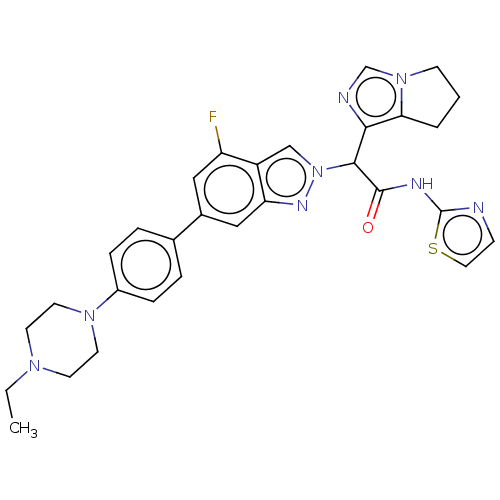
(CHEMBL5180289)Show SMILES CCN1CCN(CC1)c1ccc(cc1)-c1cc(F)c2cn(nc2c1)C(C(=O)Nc1nccs1)c1ncn2CCCc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593211
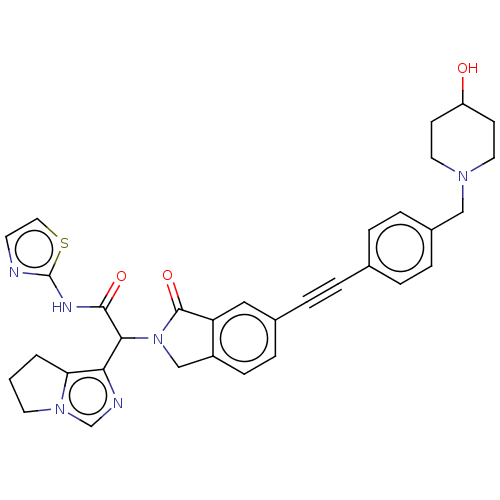
(CHEMBL5187250)Show SMILES OC1CCN(Cc2ccc(cc2)C#Cc2ccc3CN(C(C(=O)Nc4nccs4)c4ncn5CCCc45)C(=O)c3c2)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593207

(CHEMBL5189114)Show SMILES Fc1cc(cc2C(=O)N(Cc12)C(C(=O)Nc1nccs1)c1ncn2CCCc12)C#Cc1cccnc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50209753
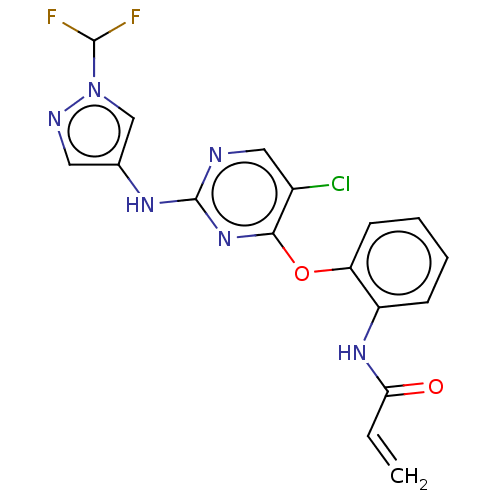
(CHEMBL3884642)Show SMILES FC(F)n1cc(Nc2ncc(Cl)c(Oc3ccccc3NC(=O)C=C)n2)cn1 Show InChI InChI=1S/C17H13ClF2N6O2/c1-2-14(27)24-12-5-3-4-6-13(12)28-15-11(18)8-21-17(25-15)23-10-7-22-26(9-10)16(19)20/h2-9,16H,1H2,(H,24,27)(H,21,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Inc
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) by LanthaScreen assay |
Bioorg Med Chem 25: 1320-1328 (2017)
Article DOI: 10.1016/j.bmc.2016.11.034
BindingDB Entry DOI: 10.7270/Q2JH3PNP |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593210
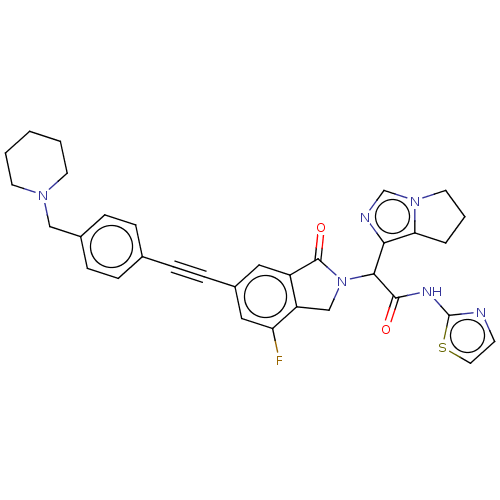
(CHEMBL5172668)Show SMILES Fc1cc(cc2C(=O)N(Cc12)C(C(=O)Nc1nccs1)c1ncn2CCCc12)C#Cc1ccc(CN2CCCCC2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50209758
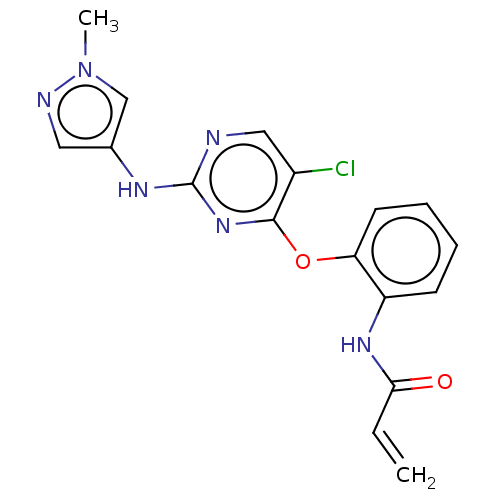
(CHEMBL3884143)Show SMILES Cn1cc(Nc2ncc(Cl)c(Oc3ccccc3NC(=O)C=C)n2)cn1 Show InChI InChI=1S/C17H15ClN6O2/c1-3-15(25)22-13-6-4-5-7-14(13)26-16-12(18)9-19-17(23-16)21-11-8-20-24(2)10-11/h3-10H,1H2,2H3,(H,22,25)(H,19,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Inc
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) by LanthaScreen assay |
Bioorg Med Chem 25: 1320-1328 (2017)
Article DOI: 10.1016/j.bmc.2016.11.034
BindingDB Entry DOI: 10.7270/Q2JH3PNP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50594428

(CHEMBL5192349)Show SMILES COc1ccc(CNC(=O)[C@@H]2CCC[C@@H](C2)Nc2ccc([N+]([O-])=O)c3nonc23)c(c1)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593227

(CHEMBL5177023)Show SMILES Cc1c(cc(C(F)F)c2cn(nc12)C(C(=O)Nc1nccs1)c1ncn2c1CCC21CC1)C#Cc1ccc(CN2CCC(CO)CC2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593223

(CHEMBL5193594)Show SMILES Cc1c(cc(C(F)F)c2cn(nc12)C(C(=O)Nc1nccs1)c1ncn2C[C@H](F)Cc12)-c1ccc(cc1)N1CCOCC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50125049
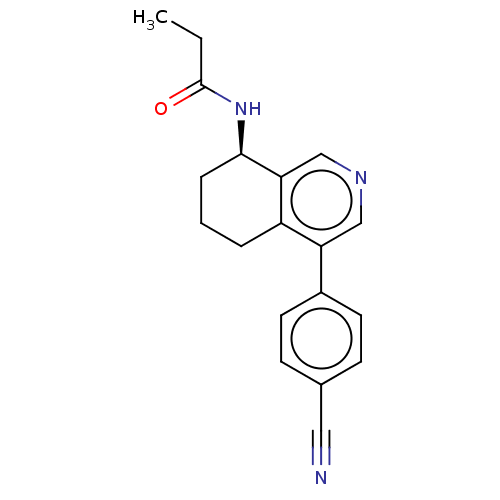
(CHEMBL3623830)Show SMILES CCC(=O)N[C@@H]1CCCc2c1cncc2-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C19H19N3O/c1-2-19(23)22-18-5-3-4-15-16(11-21-12-17(15)18)14-8-6-13(10-20)7-9-14/h6-9,11-12,18H,2-5H2,1H3,(H,22,23)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED)
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells using 11-deoxycorticosterone as substrate assessed as formation of aldoste... |
J Med Chem 58: 8054-65 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00851
BindingDB Entry DOI: 10.7270/Q2K0763C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50518965
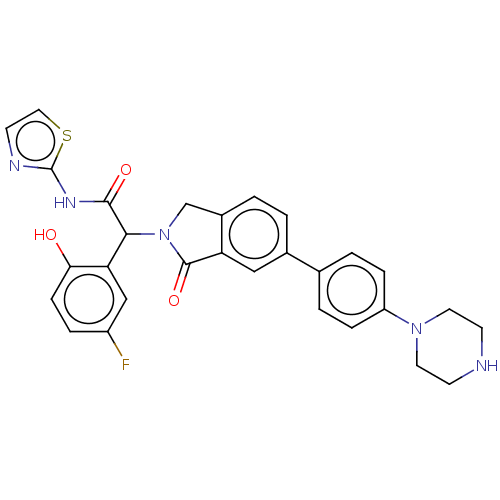
(CHEMBL4443062)Show SMILES Oc1ccc(F)cc1C(N1Cc2ccc(cc2C1=O)-c1ccc(cc1)N1CCNCC1)C(=O)Nc1nccs1 Show InChI InChI=1S/C29H26FN5O3S/c30-21-5-8-25(36)24(16-21)26(27(37)33-29-32-11-14-39-29)35-17-20-2-1-19(15-23(20)28(35)38)18-3-6-22(7-4-18)34-12-9-31-10-13-34/h1-8,11,14-16,26,31,36H,9-10,12-13,17H2,(H,32,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593224
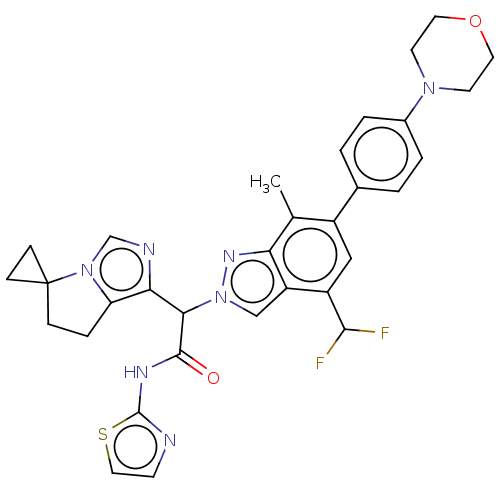
(CHEMBL5170519)Show SMILES Cc1c(cc(C(F)F)c2cn(nc12)C(C(=O)Nc1nccs1)c1ncn2c1CCC21CC1)-c1ccc(cc1)N1CCOCC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593218

(CHEMBL5203543)Show SMILES CCN1CCN(CC1)c1ccc(cc1)-c1cc(Cl)c2cn(nc2c1Cl)C(C(=O)Nc1nccs1)c1ncn2CCCc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50518965

(CHEMBL4443062)Show SMILES Oc1ccc(F)cc1C(N1Cc2ccc(cc2C1=O)-c1ccc(cc1)N1CCNCC1)C(=O)Nc1nccs1 Show InChI InChI=1S/C29H26FN5O3S/c30-21-5-8-25(36)24(16-21)26(27(37)33-29-32-11-14-39-29)35-17-20-2-1-19(15-23(20)28(35)38)18-3-6-22(7-4-18)34-12-9-31-10-13-34/h1-8,11,14-16,26,31,36H,9-10,12-13,17H2,(H,32,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593216

(CHEMBL5183324)Show SMILES CC1(C)CCc2c(ncn12)C(N1Cc2c(cc(cc2C(F)F)C#Cc2ccc(CN3CCC(CO)CC3)cc2)C1=O)C(=O)Nc1nccs1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593213

(CHEMBL5180839)Show SMILES OCC1CCN(Cc2ccc(cc2)C#Cc2cc3C(=O)N(Cc3c(F)c2)C(C(=O)Nc2nccs2)c2ncn3CCCc23)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00893
BindingDB Entry DOI: 10.7270/Q2RN3CVW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50327809

(1-(1-Propionylpiperidin-4-yl)-3-(4-(trifluorometho...)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H20F3N3O3/c1-2-14(23)22-9-7-12(8-10-22)21-15(24)20-11-3-5-13(6-4-11)25-16(17,18)19/h3-6,12H,2,7-10H2,1H3,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data