 Found 398 hits with Last Name = 'burkhart' and Initial = 'c'
Found 398 hits with Last Name = 'burkhart' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50015711
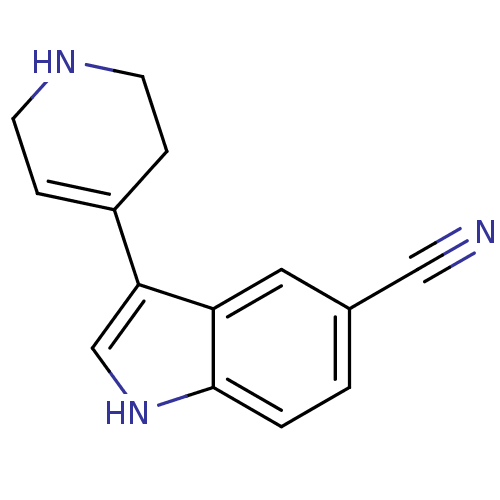
(3-(1,2,3,6-Tetrahydro-pyridin-4-yl)-1H-indole-5-ca...)Show InChI InChI=1S/C14H13N3/c15-8-10-1-2-14-12(7-10)13(9-17-14)11-3-5-16-6-4-11/h1-3,7,9,16-17H,4-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50015714
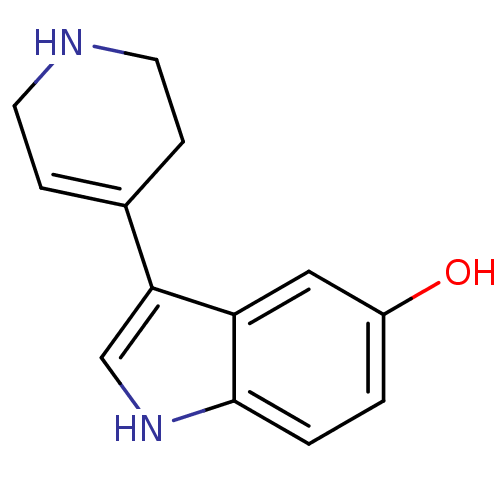
(3-(1,2,3,6-Tetrahydro-pyridin-4-yl)-1H-indol-5-ol ...)Show InChI InChI=1S/C13H14N2O/c16-10-1-2-13-11(7-10)12(8-15-13)9-3-5-14-6-4-9/h1-3,7-8,14-16H,4-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50015712

(5-Fluoro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-in...)Show InChI InChI=1S/C13H13FN2/c14-10-1-2-13-11(7-10)12(8-16-13)9-3-5-15-6-4-9/h1-3,7-8,15-16H,4-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM81498
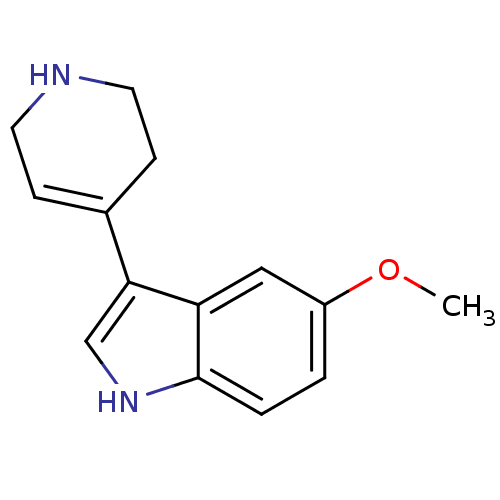
(5-Methoxy-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-i...)Show InChI InChI=1S/C14H16N2O/c1-17-11-2-3-14-12(8-11)13(9-16-14)10-4-6-15-7-5-10/h2-4,8-9,15-16H,5-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533774

(CHEMBL4469006)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cccc(c1)-c1ncnc2ccc(cc12)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H25N5O2/c1-20(36)34-11-13-35(14-12-34)30(37)24-7-4-6-23(16-24)29-26-17-21(9-10-28(26)32-19-33-29)25-15-22-5-2-3-8-27(22)31-18-25/h2-10,15-19H,11-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1D receptor of bovine caudate using [3H]-5-HT as the radioligand |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50015709
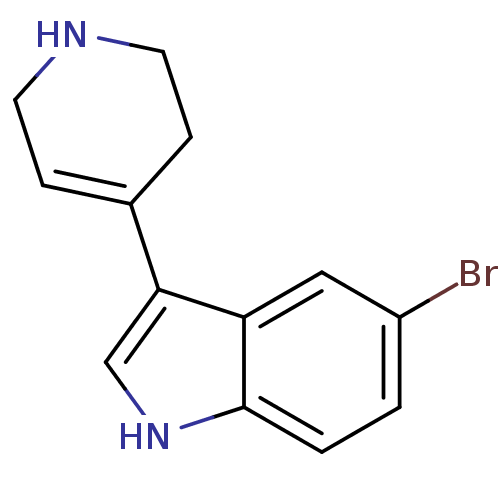
(5-Bromo-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-ind...)Show InChI InChI=1S/C13H13BrN2/c14-10-1-2-13-11(7-10)12(8-16-13)9-3-5-15-6-4-9/h1-3,7-8,15-16H,4-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50015717
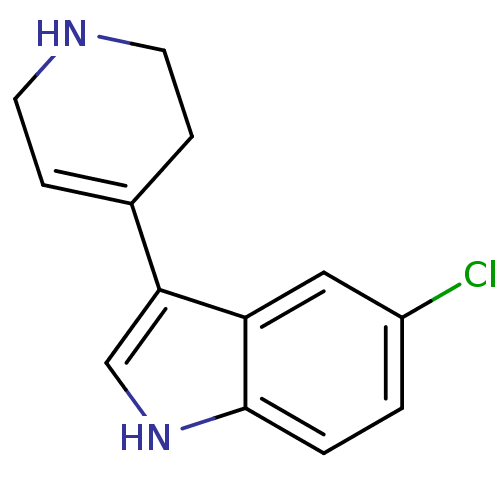
(5-Chloro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-in...)Show InChI InChI=1S/C13H13ClN2/c14-10-1-2-13-11(7-10)12(8-16-13)9-3-5-15-6-4-9/h1-3,7-8,15-16H,4-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50015718
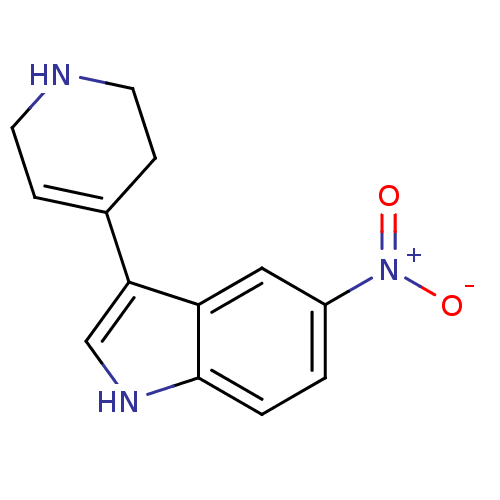
(5-Nitro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-ind...)Show SMILES [O-][N+](=O)c1ccc2[nH]cc(C3=CCNCC3)c2c1 |t:10| Show InChI InChI=1S/C13H13N3O2/c17-16(18)10-1-2-13-11(7-10)12(8-15-13)9-3-5-14-6-4-9/h1-3,7-8,14-15H,4-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM31023
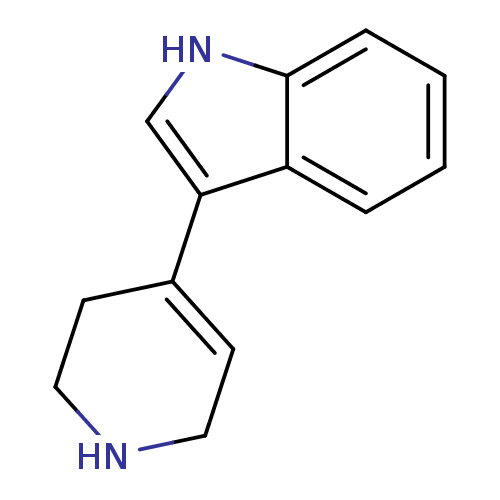
(3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole | CHE...)Show InChI InChI=1S/C13H14N2/c1-2-4-13-11(3-1)12(9-15-13)10-5-7-14-8-6-10/h1-5,9,14-15H,6-8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT as the radioligand. |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118300

(US8653092, 68)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)C3CCOCC3)c2C1 |r| Show InChI InChI=1S/C24H29F3N6O3/c1-35-22-19(24(25,26)27)10-17(11-28-22)32-7-3-20-18(13-32)21(30-14-29-20)31-16-2-6-33(12-16)23(34)15-4-8-36-9-5-15/h10-11,14-16H,2-9,12-13H2,1H3,(H,29,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins by Alexa Fluor647-labelled ADP tracer based ... |
ACS Med Chem Lett 8: 975-980 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00293
BindingDB Entry DOI: 10.7270/Q2SX6GR0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533772

(CHEMBL4521888)Show SMILES COc1ncc(cc1C(F)(F)F)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C28H24F3N5O3/c1-17(37)35-8-10-36(11-9-35)27(38)20-5-3-4-19(12-20)25-22-13-18(6-7-24(22)33-16-34-25)21-14-23(28(29,30)31)26(39-2)32-15-21/h3-7,12-16H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203680
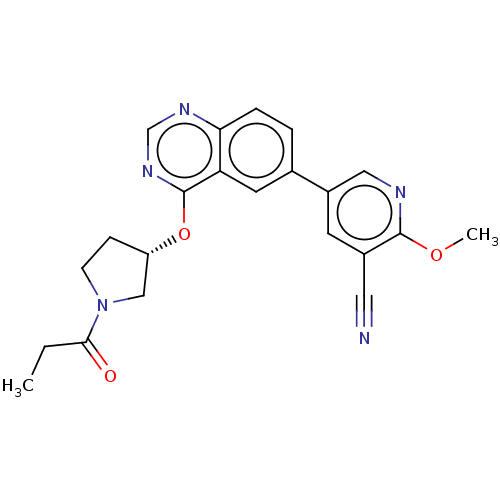
(CHEMBL3960012)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ncnc2ccc(cc12)-c1cnc(OC)c(c1)C#N |r| Show InChI InChI=1S/C22H21N5O3/c1-3-20(28)27-7-6-17(12-27)30-22-18-9-14(4-5-19(18)25-13-26-22)16-8-15(10-23)21(29-2)24-11-16/h4-5,8-9,11,13,17H,3,6-7,12H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT as the radioligand. |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human mTOR after 60 mins in presence of [gamma-33P]-ATP by microplate scintillation counting |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203699
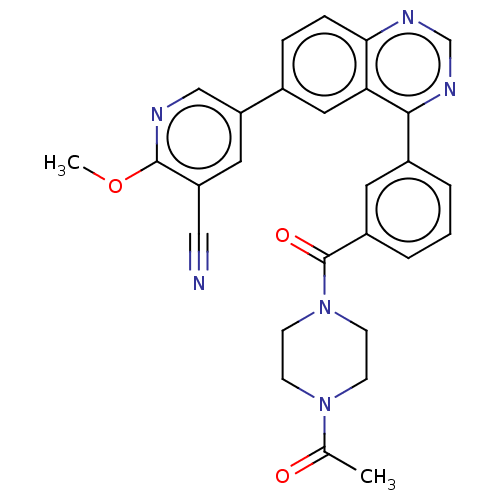
(CHEMBL3977066)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C28H24N6O3/c1-18(35)33-8-10-34(11-9-33)28(36)21-5-3-4-20(12-21)26-24-14-19(6-7-25(24)31-17-32-26)23-13-22(15-29)27(37-2)30-16-23/h3-7,12-14,16-17H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203699

(CHEMBL3977066)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C28H24N6O3/c1-18(35)33-8-10-34(11-9-33)28(36)21-5-3-4-20(12-21)26-24-14-19(6-7-25(24)31-17-32-26)23-13-22(15-29)27(37-2)30-16-23/h3-7,12-14,16-17H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533778
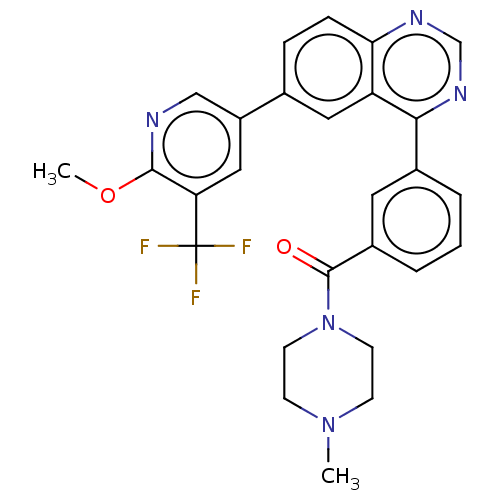
(CHEMBL4453497)Show SMILES COc1ncc(cc1C(F)(F)F)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(C)CC3)c2c1 Show InChI InChI=1S/C27H24F3N5O2/c1-34-8-10-35(11-9-34)26(36)19-5-3-4-18(12-19)24-21-13-17(6-7-23(21)32-16-33-24)20-14-22(27(28,29)30)25(37-2)31-15-20/h3-7,12-16H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203699

(CHEMBL3977066)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C28H24N6O3/c1-18(35)33-8-10-34(11-9-33)28(36)21-5-3-4-20(12-21)26-24-14-19(6-7-25(24)31-17-32-26)23-13-22(15-29)27(37-2)30-16-23/h3-7,12-14,16-17H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins by Alexa Fluor647-labelled ADP tracer based ... |
ACS Med Chem Lett 8: 975-980 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00293
BindingDB Entry DOI: 10.7270/Q2SX6GR0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203701
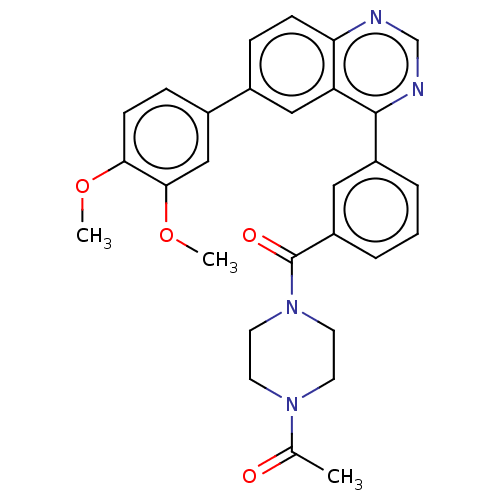
(CHEMBL3944013)Show SMILES COc1ccc(cc1OC)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C29H28N4O4/c1-19(34)32-11-13-33(14-12-32)29(35)23-6-4-5-22(15-23)28-24-16-20(7-9-25(24)30-18-31-28)21-8-10-26(36-2)27(17-21)37-3/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203696
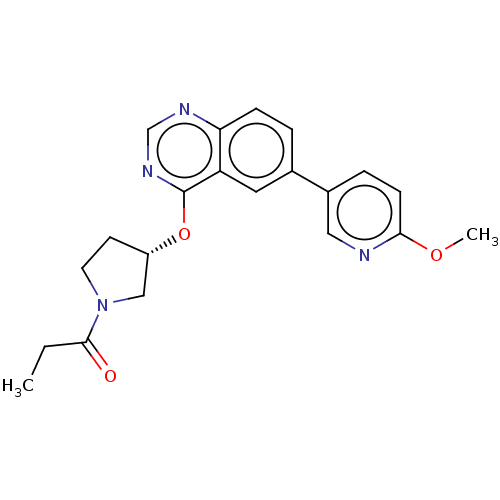
(CHEMBL3896413)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ncnc2ccc(cc12)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H22N4O3/c1-3-20(26)25-9-8-16(12-25)28-21-17-10-14(4-6-18(17)23-13-24-21)15-5-7-19(27-2)22-11-15/h4-7,10-11,13,16H,3,8-9,12H2,1-2H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Mus musculus (Mouse)) | BDBM118299

(US8653092, 67)Show SMILES CCC(=O)N1CC[C@@H](C1)Nc1ncnc2CCN(Cc12)c1cnc(OC)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H25F3N6O2/c1-3-18(31)30-6-4-13(10-30)28-19-15-11-29(7-5-17(15)26-12-27-19)14-8-16(21(22,23)24)20(32-2)25-9-14/h8-9,12-13H,3-7,10-11H2,1-2H3,(H,26,27,28)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in anti-CD3/CD28 stimulated mouse CD4-positive T cells assessed as reduction in T cell differentiation to Th2 cells by measur... |
ACS Med Chem Lett 8: 975-980 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00293
BindingDB Entry DOI: 10.7270/Q2SX6GR0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203698
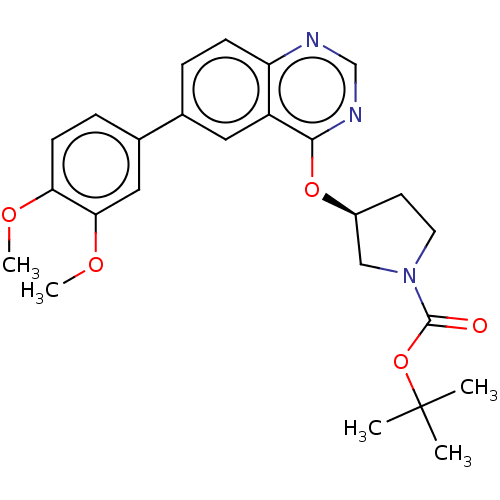
(CHEMBL3930000)Show SMILES COc1ccc(cc1OC)-c1ccc2ncnc(O[C@H]3CCN(C3)C(=O)OC(C)(C)C)c2c1 |r| Show InChI InChI=1S/C25H29N3O5/c1-25(2,3)33-24(29)28-11-10-18(14-28)32-23-19-12-16(6-8-20(19)26-15-27-23)17-7-9-21(30-4)22(13-17)31-5/h6-9,12-13,15,18H,10-11,14H2,1-5H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533778

(CHEMBL4453497)Show SMILES COc1ncc(cc1C(F)(F)F)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(C)CC3)c2c1 Show InChI InChI=1S/C27H24F3N5O2/c1-34-8-10-35(11-9-34)26(36)19-5-3-4-18(12-19)24-21-13-17(6-7-23(21)32-16-33-24)20-14-22(27(28,29)30)25(37-2)31-15-20/h3-7,12-16H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta expressed in rat-1 fibroblasts assessed as reduction in Akt phosphorylation at Ser 473 after 1 hr by alphascreen assay |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Mus musculus (Mouse)) | BDBM50203699

(CHEMBL3977066)Show SMILES COc1ncc(cc1C#N)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C28H24N6O3/c1-18(35)33-8-10-34(11-9-33)28(36)21-5-3-4-20(12-21)26-24-14-19(6-7-25(24)31-17-32-26)23-13-22(15-29)27(37-2)30-16-23/h3-7,12-14,16-17H,8-11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in Balb/c mouse in rat B cells assessed as reduction in anti-IgM induced proliferation measured over last 16 hrs of 88 hrs by... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118299

(US8653092, 67)Show SMILES CCC(=O)N1CC[C@@H](C1)Nc1ncnc2CCN(Cc12)c1cnc(OC)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H25F3N6O2/c1-3-18(31)30-6-4-13(10-30)28-19-15-11-29(7-5-17(15)26-12-27-19)14-8-16(21(22,23)24)20(32-2)25-9-14/h8-9,12-13H,3-7,10-11H2,1-2H3,(H,26,27,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins by Alexa Fluor647-labelled ADP tracer based ... |
ACS Med Chem Lett 8: 975-980 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00293
BindingDB Entry DOI: 10.7270/Q2SX6GR0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203678

(CHEMBL3905342)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ccnc2ccc(cc12)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C22H23N3O3/c1-3-22(26)25-11-9-17(14-25)28-20-8-10-23-19-6-4-15(12-18(19)20)16-5-7-21(27-2)24-13-16/h4-8,10,12-13,17H,3,9,11,14H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50015709

(5-Bromo-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-ind...)Show InChI InChI=1S/C13H13BrN2/c14-10-1-2-13-11(7-10)12(8-16-13)9-3-5-15-6-4-9/h1-3,7-8,15-16H,4-6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533772

(CHEMBL4521888)Show SMILES COc1ncc(cc1C(F)(F)F)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C28H24F3N5O3/c1-17(37)35-8-10-36(11-9-35)27(38)20-5-3-4-19(12-20)25-22-13-18(6-7-24(22)33-16-34-25)21-14-23(28(29,30)31)26(39-2)32-15-21/h3-7,12-16H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta expressed in rat-1 fibroblasts assessed as reduction in Akt phosphorylation at Ser 473 after 1 hr by alphascreen assay |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM81498

(5-Methoxy-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-i...)Show InChI InChI=1S/C14H16N2O/c1-17-11-2-3-14-12(8-11)13(9-16-14)10-4-6-15-7-5-10/h2-4,8-9,15-16H,5-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203701

(CHEMBL3944013)Show SMILES COc1ccc(cc1OC)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C29H28N4O4/c1-19(34)32-11-13-33(14-12-32)29(35)23-6-4-5-22(15-23)28-24-16-20(7-9-25(24)30-18-31-28)21-8-10-26(36-2)27(17-21)37-3/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533775

(CHEMBL4588314)Show SMILES COc1ccc(cc1OC)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(C)CC3)c2c1 Show InChI InChI=1S/C28H28N4O3/c1-31-11-13-32(14-12-31)28(33)22-6-4-5-21(15-22)27-23-16-19(7-9-24(23)29-18-30-27)20-8-10-25(34-2)26(17-20)35-3/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203685
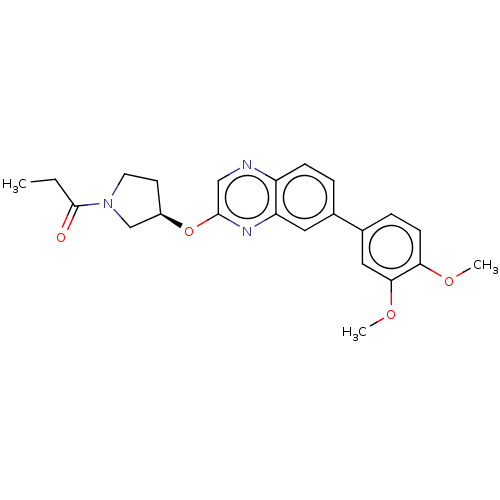
(CHEMBL3966263)Show SMILES CCC(=O)N1CC[C@H](C1)Oc1cnc2ccc(cc2n1)-c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C23H25N3O4/c1-4-23(27)26-10-9-17(14-26)30-22-13-24-18-7-5-15(11-19(18)25-22)16-6-8-20(28-2)21(12-16)29-3/h5-8,11-13,17H,4,9-10,14H2,1-3H3/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Mus musculus (Mouse)) | BDBM118300

(US8653092, 68)Show SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)C3CCOCC3)c2C1 |r| Show InChI InChI=1S/C24H29F3N6O3/c1-35-22-19(24(25,26)27)10-17(11-28-22)32-7-3-20-18(13-32)21(30-14-29-20)31-16-2-6-33(12-16)23(34)15-4-8-36-9-5-15/h10-11,14-16H,2-9,12-13H2,1H3,(H,29,30,31)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in Balb/c mouse splenocytes assessed as reduction in anti-IgM stimulated CD86 expression pretreated for 1 hr followed by anti... |
ACS Med Chem Lett 8: 975-980 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00293
BindingDB Entry DOI: 10.7270/Q2SX6GR0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM84737

(3-(1,2,3,6-Tetrahydro-pyridin-4-yl)-1,4-dihydro-py...)Show InChI InChI=1S/C12H11N3O/c16-11-2-1-10-12(15-11)9(7-14-10)8-3-5-13-6-4-8/h1-3,7,13H,4-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]5-HT |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203697
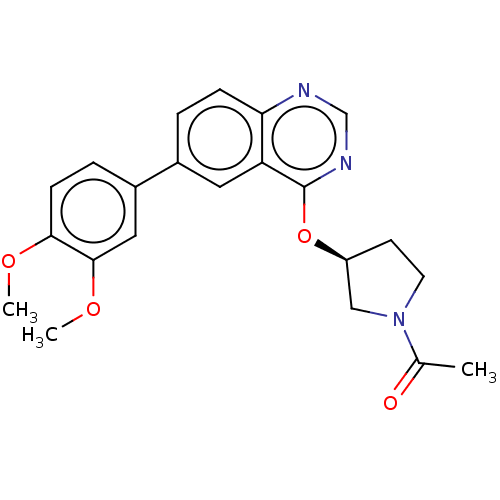
(CHEMBL3969716)Show SMILES COc1ccc(cc1OC)-c1ccc2ncnc(O[C@H]3CCN(C3)C(C)=O)c2c1 |r| Show InChI InChI=1S/C22H23N3O4/c1-14(26)25-9-8-17(12-25)29-22-18-10-15(4-6-19(18)23-13-24-22)16-5-7-20(27-2)21(11-16)28-3/h4-7,10-11,13,17H,8-9,12H2,1-3H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha (unknown origin) using phosphatidyl inositol as substrate after 30 to 60 mins in presence of ATP by Kinase Glo lu... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533775

(CHEMBL4588314)Show SMILES COc1ccc(cc1OC)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(C)CC3)c2c1 Show InChI InChI=1S/C28H28N4O3/c1-31-11-13-32(14-12-31)28(33)22-6-4-5-21(15-22)27-23-16-19(7-9-24(23)29-18-30-27)20-8-10-25(34-2)26(17-20)35-3/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta expressed in rat-1 fibroblasts assessed as reduction in Akt phosphorylation at Ser 473 after 1 hr by alphascreen assay |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM81497
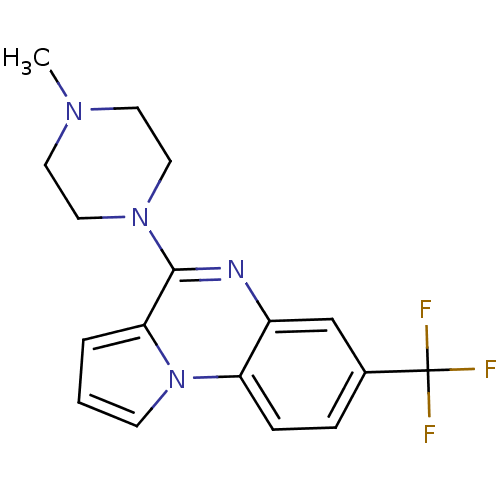
(4-(4-Methyl-piperazin-1-yl)-7-trifluoromethyl-pyrr...)Show InChI InChI=1S/C17H17F3N4/c1-22-7-9-23(10-8-22)16-15-3-2-6-24(15)14-5-4-12(17(18,19)20)11-13(14)21-16/h2-6,11H,7-10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533776

(CHEMBL4581278)Show SMILES COc1ccc(cn1)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(C)CC3)c2c1 Show InChI InChI=1S/C26H25N5O2/c1-30-10-12-31(13-11-30)26(32)20-5-3-4-19(14-20)25-22-15-18(6-8-23(22)28-17-29-25)21-7-9-24(33-2)27-16-21/h3-9,14-17H,10-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118313
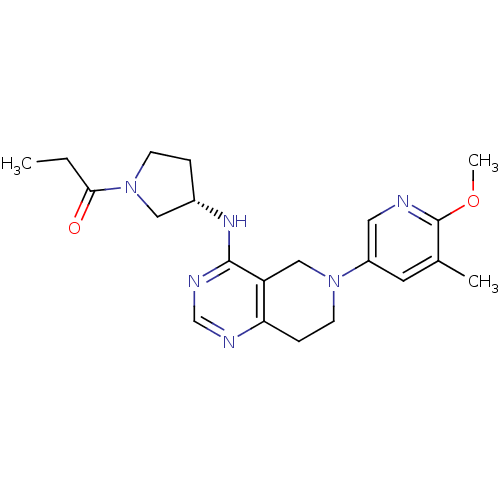
(US8653092, 81)Show SMILES CCC(=O)N1CC[C@@H](C1)Nc1ncnc2CCN(Cc12)c1cnc(OC)c(C)c1 |r| Show InChI InChI=1S/C21H28N6O2/c1-4-19(28)27-7-5-15(11-27)25-20-17-12-26(8-6-18(17)23-13-24-20)16-9-14(2)21(29-3)22-10-16/h9-10,13,15H,4-8,11-12H2,1-3H3,(H,23,24,25)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins by Alexa Fluor647-labelled ADP tracer based ... |
ACS Med Chem Lett 8: 975-980 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00293
BindingDB Entry DOI: 10.7270/Q2SX6GR0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203682
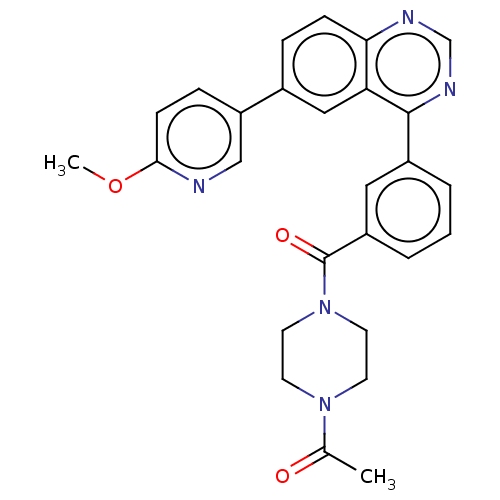
(CHEMBL3947814)Show SMILES COc1ccc(cn1)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(CC3)C(C)=O)c2c1 Show InChI InChI=1S/C27H25N5O3/c1-18(33)31-10-12-32(13-11-31)27(34)21-5-3-4-20(14-21)26-23-15-19(6-8-24(23)29-17-30-26)22-7-9-25(35-2)28-16-22/h3-9,14-17H,10-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50015710

(4-Piperazin-1-yl-7-trifluoromethyl-pyrrolo[1,2-a]q...)Show InChI InChI=1S/C16H15F3N4/c17-16(18,19)11-3-4-13-12(10-11)21-15(14-2-1-7-23(13)14)22-8-5-20-6-9-22/h1-4,7,10,20H,5-6,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50203677

(CHEMBL3977135)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ccnc2ccc(nc12)-c1ccc(OC)nc1 |r| Show InChI InChI=1S/C21H22N4O3/c1-3-20(26)25-11-9-15(13-25)28-18-8-10-22-17-6-5-16(24-21(17)18)14-4-7-19(27-2)23-12-14/h4-8,10,12,15H,3,9,11,13H2,1-2H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-delta (unknown origin) by KinaseGlo assay |
Bioorg Med Chem Lett 26: 5657-5662 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.069
BindingDB Entry DOI: 10.7270/Q20Z7574 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50015717

(5-Chloro-3-(1,2,3,6-tetrahydro-pyridin-4-yl)-1H-in...)Show InChI InChI=1S/C13H13ClN2/c14-10-1-2-13-11(7-10)12(8-16-13)9-3-5-15-6-4-9/h1-3,7-8,15-16H,4-6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from rat cortex 5-hydroxytryptamine 1A receptor |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Mus musculus (Mouse)) | BDBM50533775

(CHEMBL4588314)Show SMILES COc1ccc(cc1OC)-c1ccc2ncnc(-c3cccc(c3)C(=O)N3CCN(C)CC3)c2c1 Show InChI InChI=1S/C28H28N4O3/c1-31-11-13-32(14-12-31)28(33)22-6-4-5-21(15-22)27-23-16-19(7-9-24(23)29-18-30-27)20-8-10-25(34-2)26(17-20)35-3/h4-10,15-18H,11-14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in Balb/c mouse B cells assessed as reduction in anti-IgM induced CD86 expression preincubated for 1 hr followed by anti-IgM ... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM118295

(US8653092, 63)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ncnc2CCN(Cc12)c1cnc(OC)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H24F3N5O3/c1-3-18(30)29-6-4-14(10-29)32-19-15-11-28(7-5-17(15)26-12-27-19)13-8-16(21(22,23)24)20(31-2)25-9-13/h8-9,12,14H,3-7,10-11H2,1-2H3/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of human platelet aggregation induced by alpha-thrombin (at a concentration of 0.15 nM) |
ACS Med Chem Lett 8: 975-980 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00293
BindingDB Entry DOI: 10.7270/Q2SX6GR0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50007406
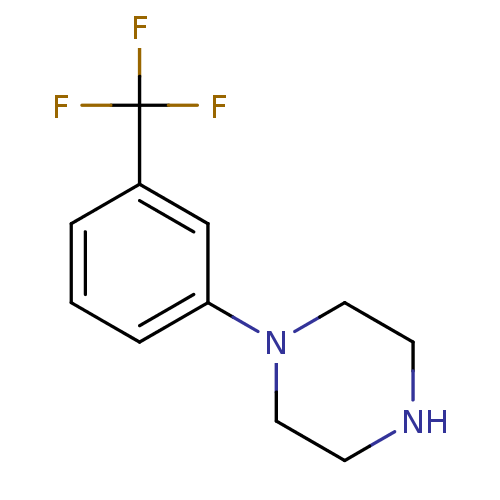
(1-(3-(trifluoromethyl)phenyl)piperazine | 1-(3-Tri...)Show InChI InChI=1S/C11H13F3N2/c12-11(13,14)9-2-1-3-10(8-9)16-6-4-15-5-7-16/h1-3,8,15H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat cortex using [3H]-5-HT |
J Med Chem 33: 2087-93 (1990)
BindingDB Entry DOI: 10.7270/Q2GQ6WR3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data