

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM12983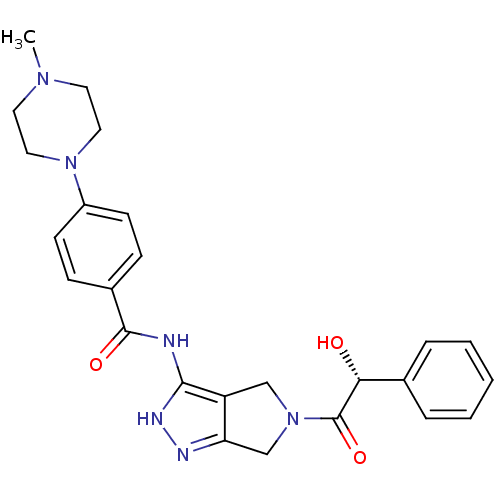 (5-Amido-pyrrolopyrazole 9b | CHEMBL385872 | N-{5-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12982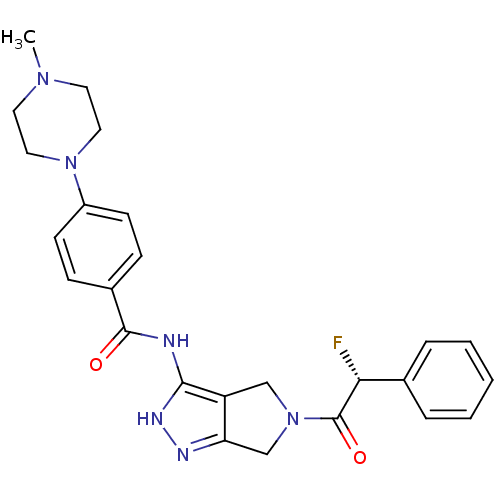 (5-Amido-pyrrolopyrazole 9a | CHEMBL385266 | N-{5-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12985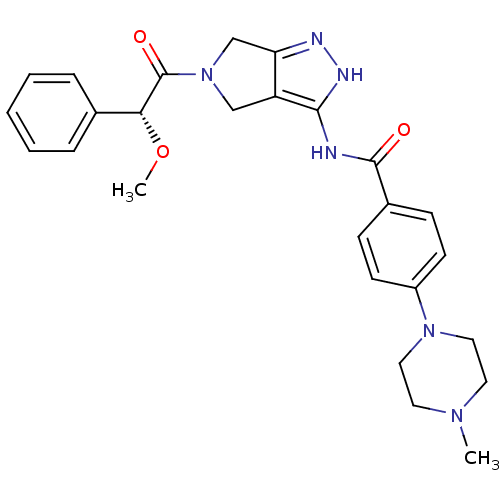 (5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12984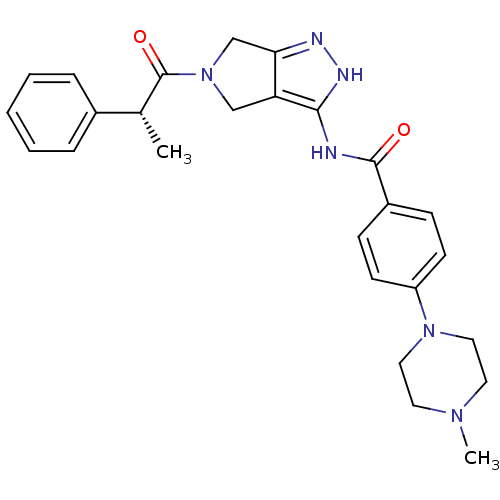 (4-(4-methylpiperazin-1-yl)-N-{5-[(2R)-2-phenylprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12110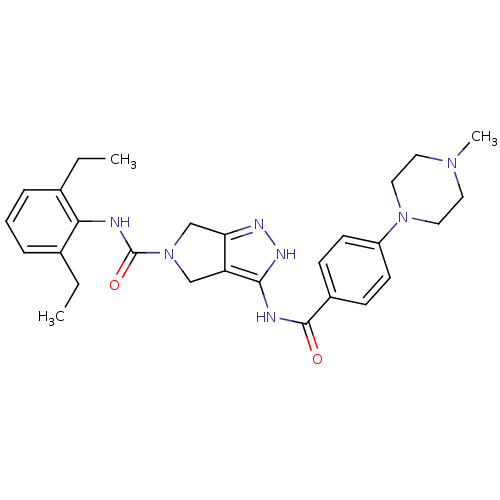 (1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 18 | 5-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM12983 (5-Amido-pyrrolopyrazole 9b | CHEMBL385872 | N-{5-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12107 (1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 15 | 4-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM12985 (5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM12984 (4-(4-methylpiperazin-1-yl)-N-{5-[(2R)-2-phenylprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12109 (1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 17 | 4-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM12982 (5-Amido-pyrrolopyrazole 9a | CHEMBL385266 | N-{5-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12987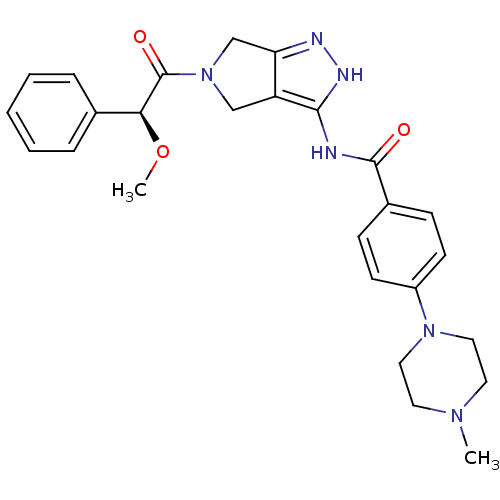 (5-Amido-pyrrolopyrazole 9f | CHEMBL384575 | N-{5-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 354 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12986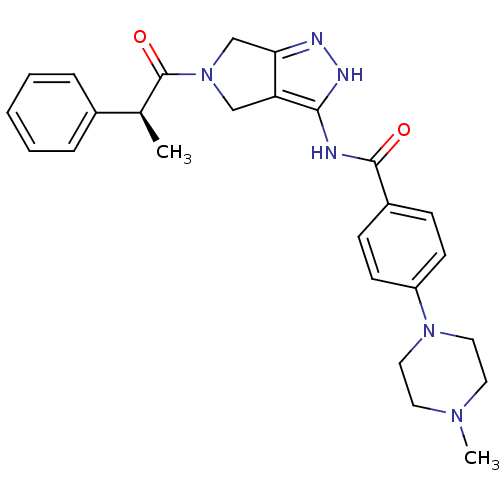 (4-(4-methylpiperazin-1-yl)-N-{5-[(2S)-2-phenylprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 452 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM12987 (5-Amido-pyrrolopyrazole 9f | CHEMBL384575 | N-{5-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM12986 (4-(4-methylpiperazin-1-yl)-N-{5-[(2S)-2-phenylprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50008936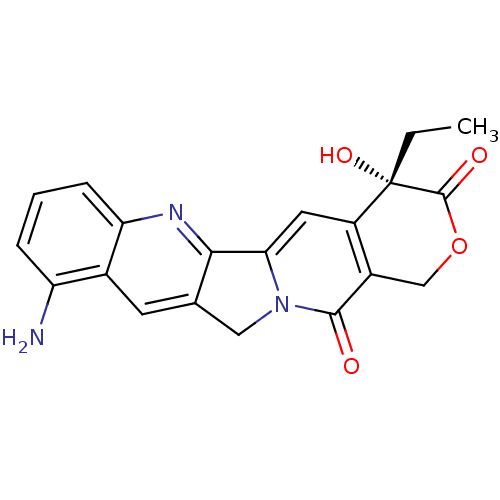 ((S)-10-Amino-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50288994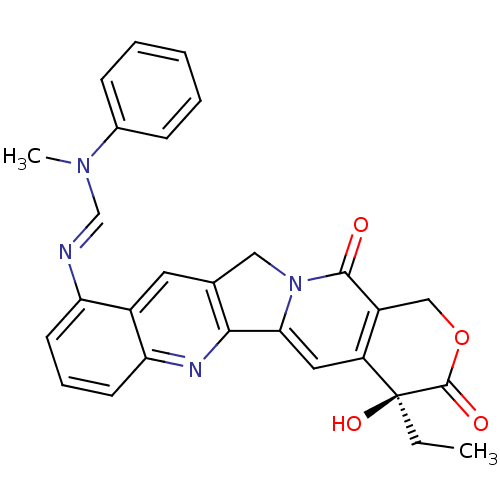 (CHEMBL545578 | N'-((S)-4-Ethyl-4-hydroxy-3,13-diox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of topoisomerase I. | Bioorg Med Chem Lett 6: 671-674 (1996) Article DOI: 10.1016/0960-894X(96)00083-2 BindingDB Entry DOI: 10.7270/Q2T153MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291417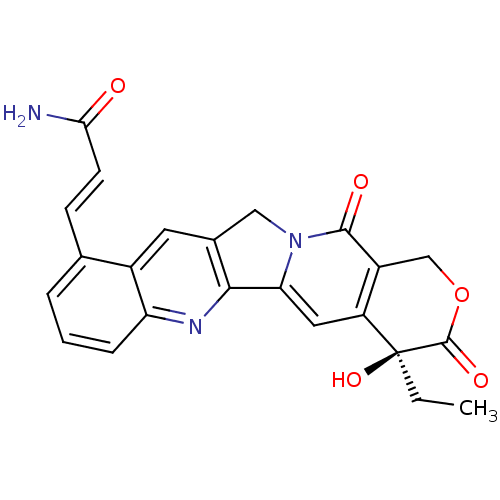 ((E)-3-((S)-4-Ethyl-4-hydroxy-3,13-dioxo-3,4,12,13-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50288991 (CHEMBL555409 | N'-((S)-4-Ethyl-4-hydroxy-3,13-diox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of topoisomerase I. | Bioorg Med Chem Lett 6: 671-674 (1996) Article DOI: 10.1016/0960-894X(96)00083-2 BindingDB Entry DOI: 10.7270/Q2T153MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50288989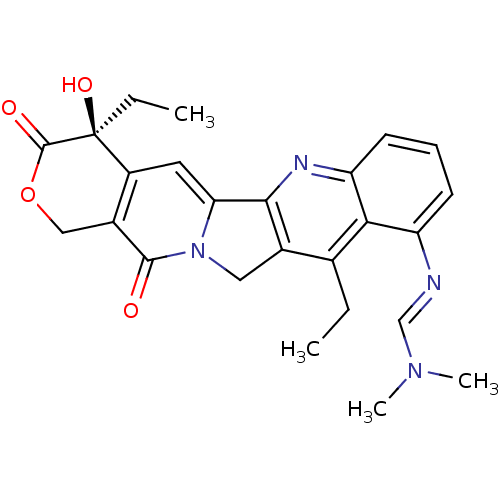 (CHEMBL540086 | N'-((S)-4,11-Diethyl-4-hydroxy-3,13...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of topoisomerase I. | Bioorg Med Chem Lett 6: 671-674 (1996) Article DOI: 10.1016/0960-894X(96)00083-2 BindingDB Entry DOI: 10.7270/Q2T153MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291415 ((S)-4-Ethyl-4-hydroxy-10-vinyl-1,12-dihydro-4H-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50008936 ((S)-10-Amino-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of topoisomerase I. | Bioorg Med Chem Lett 6: 671-674 (1996) Article DOI: 10.1016/0960-894X(96)00083-2 BindingDB Entry DOI: 10.7270/Q2T153MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50288988 (CHEMBL542775 | N-((S)-4-Ethyl-4-hydroxy-3,13-dioxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of topoisomerase I. | Bioorg Med Chem Lett 6: 671-674 (1996) Article DOI: 10.1016/0960-894X(96)00083-2 BindingDB Entry DOI: 10.7270/Q2T153MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291418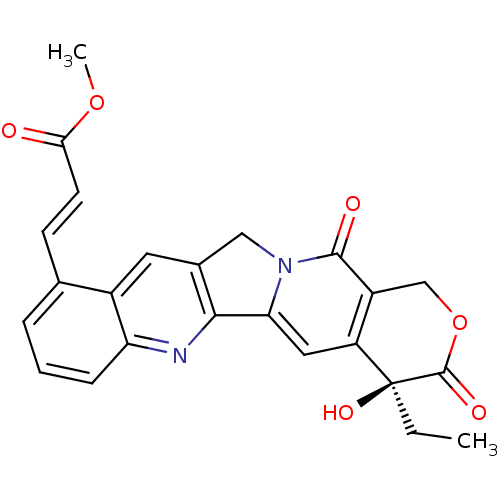 ((E)-3-((S)-4-Ethyl-4-hydroxy-3,13-dioxo-3,4,12,13-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291416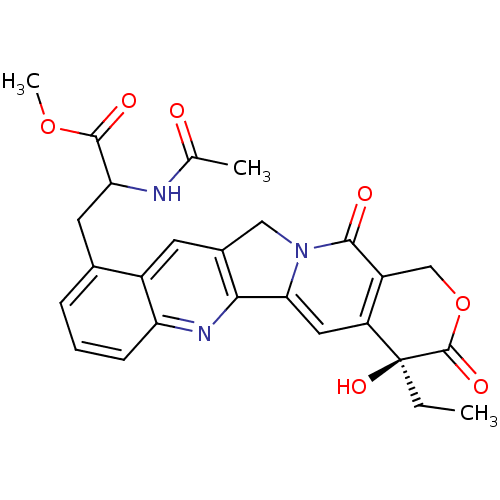 (2-Acetylamino-3-((S)-4-ethyl-4-hydroxy-3,13-dioxo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50288993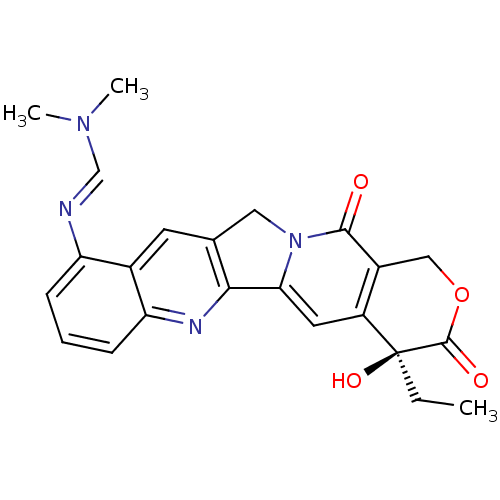 (CHEMBL543237 | N'-((S)-4-Ethyl-4-hydroxy-3,13-diox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of topoisomerase I. | Bioorg Med Chem Lett 6: 671-674 (1996) Article DOI: 10.1016/0960-894X(96)00083-2 BindingDB Entry DOI: 10.7270/Q2T153MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50288992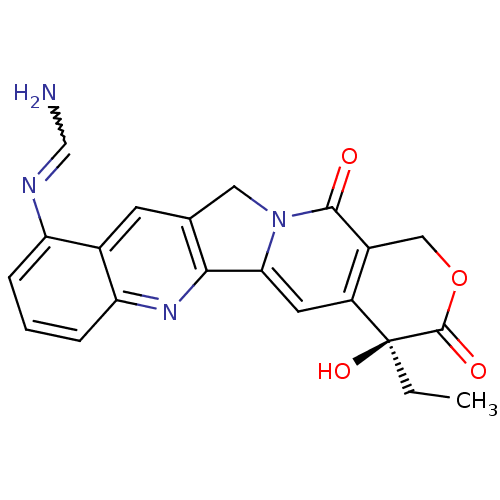 (CHEMBL543000 | N-((S)-4-Ethyl-4-hydroxy-3,13-dioxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of topoisomerase I. | Bioorg Med Chem Lett 6: 671-674 (1996) Article DOI: 10.1016/0960-894X(96)00083-2 BindingDB Entry DOI: 10.7270/Q2T153MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291412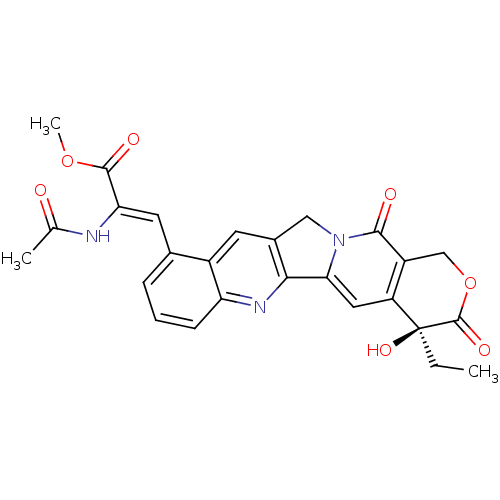 ((Z)-2-Acetylamino-3-((S)-4-ethyl-4-hydroxy-3,13-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291410 (3-((S)-4-Ethyl-4-hydroxy-3,13-dioxo-3,4,12,13-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50288995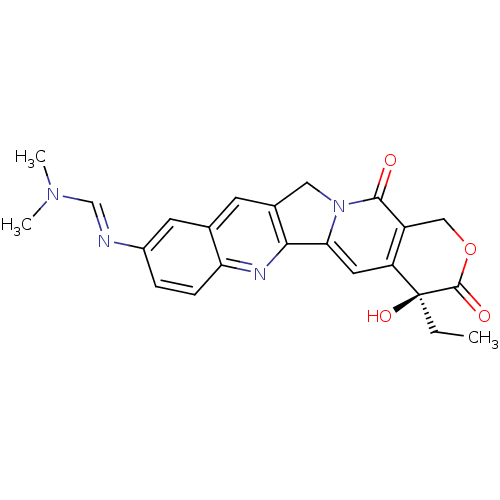 (CHEMBL554038 | N'-((S)-4-Ethyl-4-hydroxy-3,13-diox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of topoisomerase I. | Bioorg Med Chem Lett 6: 671-674 (1996) Article DOI: 10.1016/0960-894X(96)00083-2 BindingDB Entry DOI: 10.7270/Q2T153MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50288990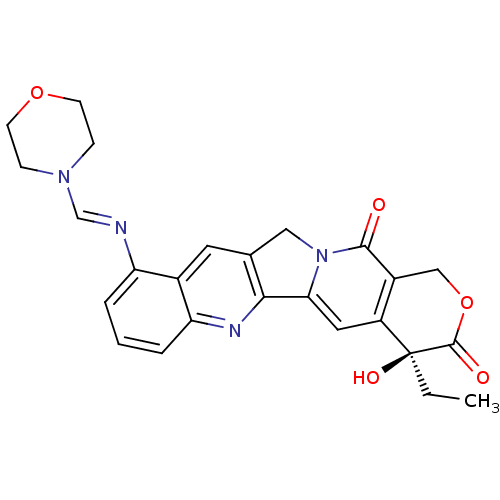 ((S)-4-Ethyl-4-hydroxy-10-{[1-morpholin-4-yl-meth-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of topoisomerase I. | Bioorg Med Chem Lett 6: 671-674 (1996) Article DOI: 10.1016/0960-894X(96)00083-2 BindingDB Entry DOI: 10.7270/Q2T153MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291414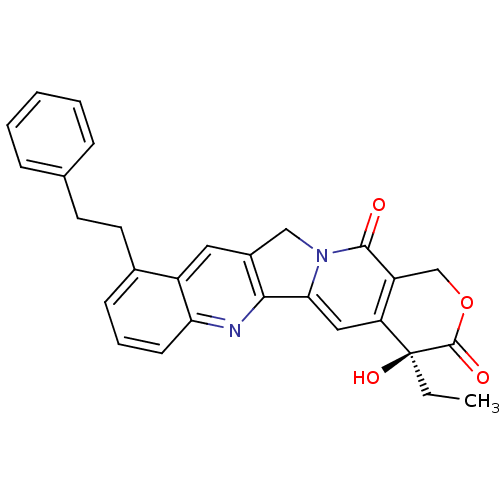 ((S)-4-Ethyl-4-hydroxy-10-phenethyl-1,12-dihydro-4H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291411 ((S)-4-Ethyl-4-hydroxy-10-((E)-styryl)-1,12-dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291413 ((E)-3-((S)-4-Ethyl-4-hydroxy-3,13-dioxo-3,4,12,13-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||