

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM92520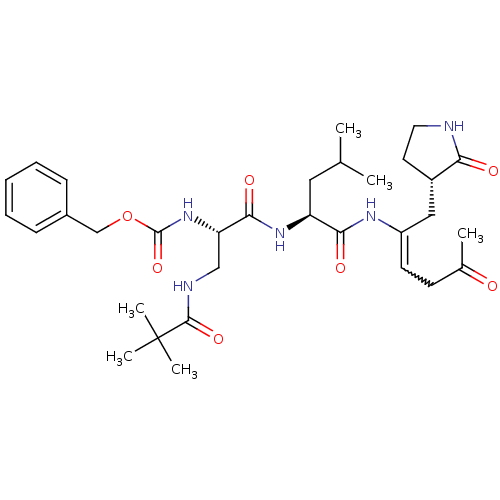 (TG-0204998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | -42.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | -41.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232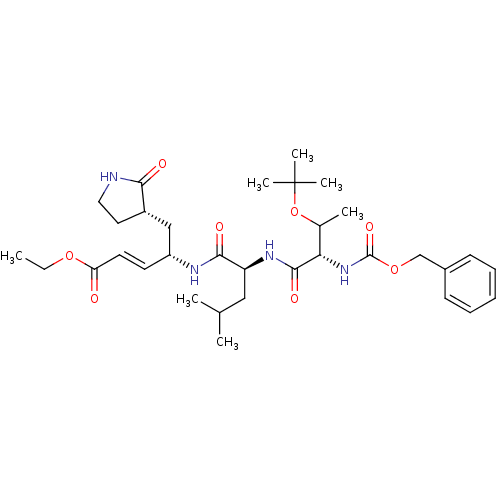 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM92521 (TG-0205486) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 99 | -40.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM92521 (TG-0205486) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -36.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11231 (N-[(benzyloxy)carbonyl]-L-valyl-N1-((1S,2E)-4-etho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 660 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM92520 (TG-0204998) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | -34.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM11232 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230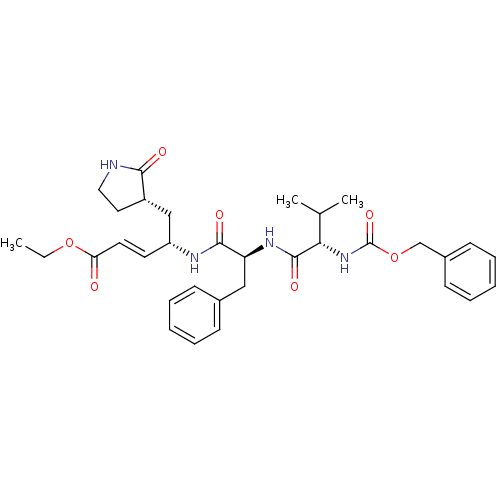 (AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.26E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Coxsackievirus B3 (strain Nancy)) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
National Yang-Ming University | Assay Description Peptidomimetic inhibitors against CVB3 3Cpro and SARS-CoV 3CLpro. The inhibition constant of SARS 3CLpro was analyzed with reverse-phase HPLC using a... | J Biol Chem 284: 7646-55 (2009) Article DOI: 10.1074/jbc.M807947200 BindingDB Entry DOI: 10.7270/Q2SN07KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11229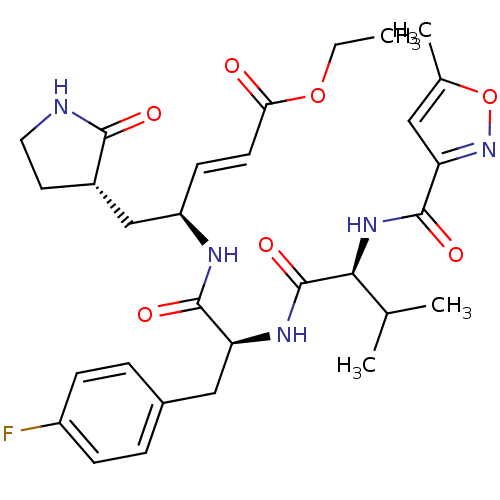 (AG7088 analogue 2a | CHEMBL20636 | N-[(5-methyliso...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein ENL (Homo sapiens) | BDBM50529556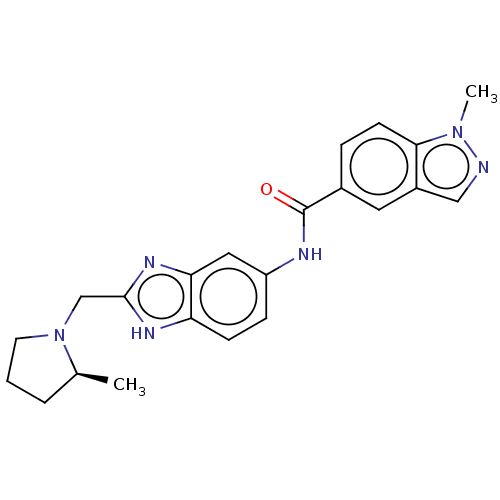 (CHEMBL4552313) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00775 BindingDB Entry DOI: 10.7270/Q2W381BN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein ENL (Homo sapiens) | BDBM50596391 (CHEMBL5171384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00775 BindingDB Entry DOI: 10.7270/Q2W381BN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein ENL (Homo sapiens) | BDBM50596391 (CHEMBL5171384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00775 BindingDB Entry DOI: 10.7270/Q2W381BN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||