 Found 360 hits with Last Name = 'chiba' and Initial = 't'
Found 360 hits with Last Name = 'chiba' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031226
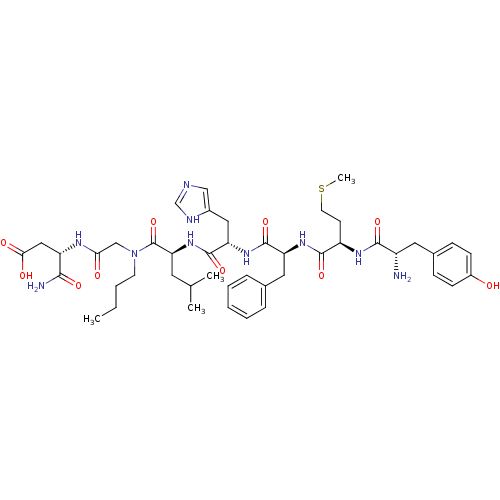
((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CCCCN(CC(=O)N[C@@H](CC(O)=O)C(N)=O)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C45H64N10O10S/c1-5-6-17-55(25-38(57)50-34(40(47)60)23-39(58)59)45(65)37(19-27(2)3)54-44(64)36(22-30-24-48-26-49-30)53-43(63)35(21-28-10-8-7-9-11-28)52-42(62)33(16-18-66-4)51-41(61)32(46)20-29-12-14-31(56)15-13-29/h7-15,24,26-27,32-37,56H,5-6,16-23,25,46H2,1-4H3,(H2,47,60)(H,48,49)(H,50,57)(H,51,61)(H,52,62)(H,53,63)(H,54,64)(H,58,59)/t32-,33+,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DADLE binding to rat brain delta receptor |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031219
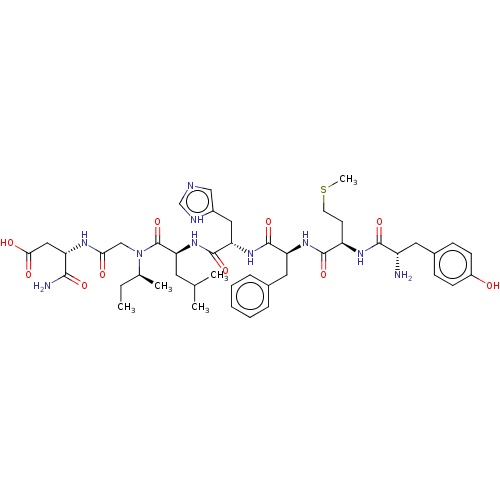
((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CC[C@H](C)N(CC(=O)N[C@@H](CC(O)=O)C(N)=O)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C45H64N10O10S/c1-6-27(4)55(24-38(57)50-34(40(47)60)22-39(58)59)45(65)37(18-26(2)3)54-44(64)36(21-30-23-48-25-49-30)53-43(63)35(20-28-10-8-7-9-11-28)52-42(62)33(16-17-66-5)51-41(61)32(46)19-29-12-14-31(56)15-13-29/h7-15,23,25-27,32-37,56H,6,16-22,24,46H2,1-5H3,(H2,47,60)(H,48,49)(H,50,57)(H,51,61)(H,52,62)(H,53,63)(H,54,64)(H,58,59)/t27-,32-,33+,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DADLE binding to rat brain delta receptor |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50126170

(CHEMBL3629678)Show SMILES CCCCCCCCCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1\C=C\CCNC(=O)\C=C\[C@@H](NC1=O)C(C)C |r,t:25,32| Show InChI InChI=1S/C32H48N4O4/c1-4-5-6-7-8-9-13-19-30(38)34-28(23-25-16-11-10-12-17-25)32(40)36-27-18-14-15-22-33-29(37)21-20-26(24(2)3)35-31(27)39/h10-12,14,16-18,20-21,24,26-28H,4-9,13,15,19,22-23H2,1-3H3,(H,33,37)(H,34,38)(H,35,39)(H,36,40)/b18-14+,21-20+/t26-,27+,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of proteasome beta-5 (unknown origin) |
Bioorg Med Chem Lett 25: 4872-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.015
BindingDB Entry DOI: 10.7270/Q2028TBR |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009180
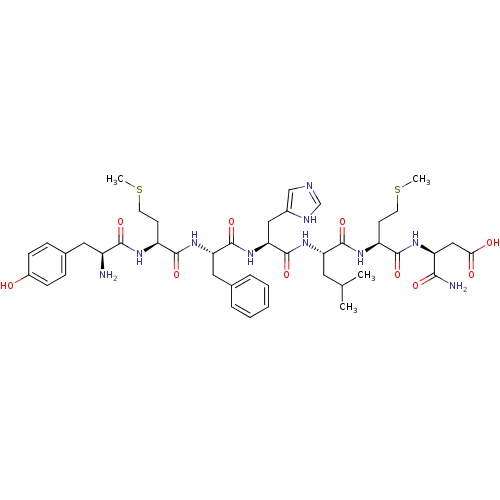
(3-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-pro...)Show SMILES CSCC[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C44H62N10O10S2/c1-25(2)18-34(42(62)50-32(15-17-66-4)40(60)51-33(38(46)58)22-37(56)57)52-44(64)36(21-28-23-47-24-48-28)54-43(63)35(20-26-8-6-5-7-9-26)53-41(61)31(14-16-65-3)49-39(59)30(45)19-27-10-12-29(55)13-11-27/h5-13,23-25,30-36,55H,14-22,45H2,1-4H3,(H2,46,58)(H,47,48)(H,49,59)(H,50,62)(H,51,60)(H,52,64)(H,53,61)(H,54,63)(H,56,57)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DADLE binding to rat brain delta receptor |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031222

((S)-3-((S)-2-{(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Am...)Show SMILES CSCC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(=O)N[C@@H](CC(O)=O)C(N)=O)C(C)(C)C Show InChI InChI=1S/C45H64N10O10S/c1-25(2)18-33(43(64)55-37(45(3,4)5)44(65)51-32(38(47)59)22-36(57)58)52-42(63)35(21-28-23-48-24-49-28)54-41(62)34(20-26-10-8-7-9-11-26)53-40(61)31(16-17-66-6)50-39(60)30(46)19-27-12-14-29(56)15-13-27/h7-15,23-25,30-35,37,56H,16-22,46H2,1-6H3,(H2,47,59)(H,48,49)(H,50,60)(H,51,65)(H,52,63)(H,53,61)(H,54,62)(H,55,64)(H,57,58)/t30-,31+,32-,33-,34-,35-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DADLE binding to rat brain delta receptor |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031218
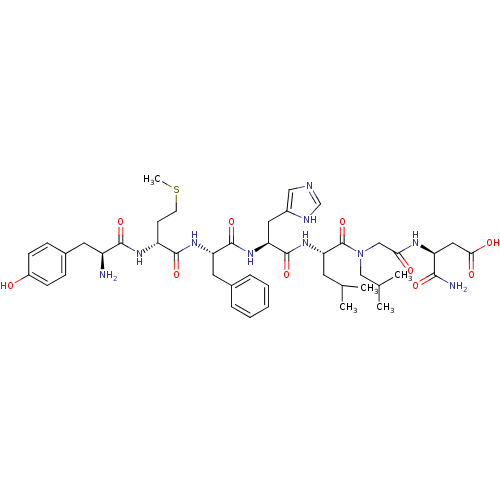
((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CSCC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(C)C)C(=O)N(CC(C)C)CC(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C45H64N10O10S/c1-26(2)17-37(45(65)55(23-27(3)4)24-38(57)50-34(40(47)60)21-39(58)59)54-44(64)36(20-30-22-48-25-49-30)53-43(63)35(19-28-9-7-6-8-10-28)52-42(62)33(15-16-66-5)51-41(61)32(46)18-29-11-13-31(56)14-12-29/h6-14,22,25-27,32-37,56H,15-21,23-24,46H2,1-5H3,(H2,47,60)(H,48,49)(H,50,57)(H,51,61)(H,52,62)(H,53,63)(H,54,64)(H,58,59)/t32-,33+,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DADLE binding to rat brain delta receptor |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031224

((S)-3-((S)-2-{(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Am...)Show SMILES CSCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C41H56N10O10S2/c1-23(36(56)47-29(13-15-62-2)38(58)49-31(35(43)55)20-34(53)54)46-40(60)33(19-26-21-44-22-45-26)51-41(61)32(18-24-7-5-4-6-8-24)50-39(59)30(14-16-63-3)48-37(57)28(42)17-25-9-11-27(52)12-10-25/h4-12,21-23,28-33,52H,13-20,42H2,1-3H3,(H2,43,55)(H,44,45)(H,46,60)(H,47,56)(H,48,57)(H,49,58)(H,50,59)(H,51,61)(H,53,54)/t23-,28-,29-,30+,31-,32-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DADLE binding to rat brain delta receptor |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031225
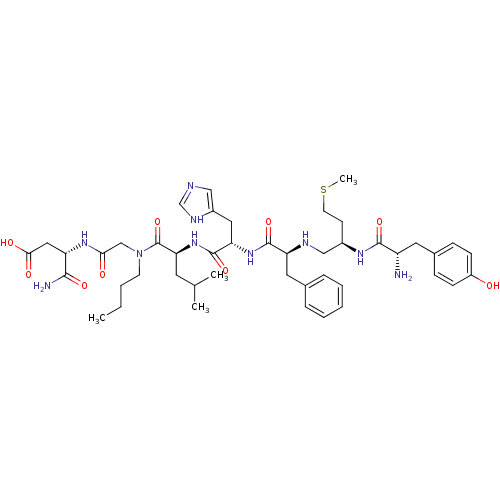
((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CCCCN(CC(=O)N[C@@H](CC(O)=O)C(N)=O)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C45H66N10O9S/c1-5-6-17-55(26-39(57)52-35(41(47)60)23-40(58)59)45(64)38(19-28(2)3)54-44(63)37(22-32-24-48-27-50-32)53-43(62)36(21-29-10-8-7-9-11-29)49-25-31(16-18-65-4)51-42(61)34(46)20-30-12-14-33(56)15-13-30/h7-15,24,27-28,31,34-38,49,56H,5-6,16-23,25-26,46H2,1-4H3,(H2,47,60)(H,48,50)(H,51,61)(H,52,57)(H,53,62)(H,54,63)(H,58,59)/t31-,34+,35+,36+,37+,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DADLE binding to rat brain delta receptor |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031221
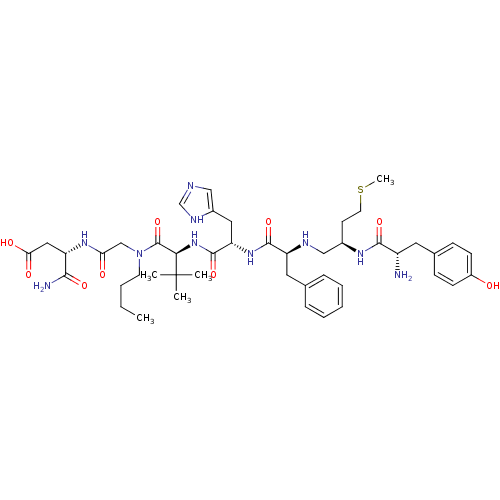
((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CCCCN(CC(=O)N[C@@H](CC(O)=O)C(N)=O)C(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)(C)C Show InChI InChI=1S/C45H66N10O9S/c1-6-7-18-55(26-37(57)52-34(40(47)60)23-38(58)59)44(64)39(45(2,3)4)54-43(63)36(22-31-24-48-27-50-31)53-42(62)35(21-28-11-9-8-10-12-28)49-25-30(17-19-65-5)51-41(61)33(46)20-29-13-15-32(56)16-14-29/h8-16,24,27,30,33-36,39,49,56H,6-7,17-23,25-26,46H2,1-5H3,(H2,47,60)(H,48,50)(H,51,61)(H,52,57)(H,53,62)(H,54,63)(H,58,59)/t30-,33+,34+,35+,36+,39-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DADLE binding to rat brain delta receptor |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031220

(3-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-pro...)Show SMILES CSCC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(N)=O)C(C)(C)C Show InChI InChI=1S/C44H62N10O10S2/c1-44(2,3)36(43(64)50-31(16-18-66-5)39(60)51-32(37(46)58)22-35(56)57)54-42(63)34(21-27-23-47-24-48-27)53-41(62)33(20-25-9-7-6-8-10-25)52-40(61)30(15-17-65-4)49-38(59)29(45)19-26-11-13-28(55)14-12-26/h6-14,23-24,29-34,36,55H,15-22,45H2,1-5H3,(H2,46,58)(H,47,48)(H,49,59)(H,50,64)(H,51,60)(H,52,61)(H,53,62)(H,54,63)(H,56,57)/t29-,30+,31-,32-,33-,34-,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DADLE binding to rat brain delta receptor |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031223
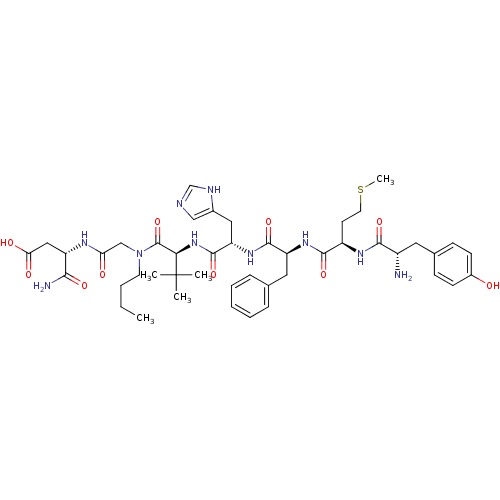
((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CCCCN(CC(=O)N[C@@H](CC(O)=O)C(N)=O)C(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)(C)C Show InChI InChI=1S/C45H64N10O10S/c1-6-7-18-55(25-36(57)50-33(39(47)60)23-37(58)59)44(65)38(45(2,3)4)54-43(64)35(22-29-24-48-26-49-29)53-42(63)34(21-27-11-9-8-10-12-27)52-41(62)32(17-19-66-5)51-40(61)31(46)20-28-13-15-30(56)16-14-28/h8-16,24,26,31-35,38,56H,6-7,17-23,25,46H2,1-5H3,(H2,47,60)(H,48,49)(H,50,57)(H,51,61)(H,52,62)(H,53,63)(H,54,64)(H,58,59)/t31-,32+,33-,34-,35-,38+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DADLE binding to rat brain delta receptor |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031218

((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CSCC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(C)C)C(=O)N(CC(C)C)CC(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C45H64N10O10S/c1-26(2)17-37(45(65)55(23-27(3)4)24-38(57)50-34(40(47)60)21-39(58)59)54-44(64)36(20-30-22-48-25-49-30)53-43(63)35(19-28-9-7-6-8-10-28)52-42(62)33(15-16-66-5)51-41(61)32(46)18-29-11-13-31(56)14-12-29/h6-14,22,25-27,32-37,56H,15-21,23-24,46H2,1-5H3,(H2,47,60)(H,48,49)(H,50,57)(H,51,61)(H,52,62)(H,53,63)(H,54,64)(H,58,59)/t32-,33+,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAGO binding to rat brain Opioid receptor mu 1 |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031224

((S)-3-((S)-2-{(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Am...)Show SMILES CSCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C41H56N10O10S2/c1-23(36(56)47-29(13-15-62-2)38(58)49-31(35(43)55)20-34(53)54)46-40(60)33(19-26-21-44-22-45-26)51-41(61)32(18-24-7-5-4-6-8-24)50-39(59)30(14-16-63-3)48-37(57)28(42)17-25-9-11-27(52)12-10-25/h4-12,21-23,28-33,52H,13-20,42H2,1-3H3,(H2,43,55)(H,44,45)(H,46,60)(H,47,56)(H,48,57)(H,49,58)(H,50,59)(H,51,61)(H,53,54)/t23-,28-,29-,30+,31-,32-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAGO binding to rat brain Opioid receptor mu 1 |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031225

((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CCCCN(CC(=O)N[C@@H](CC(O)=O)C(N)=O)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C45H66N10O9S/c1-5-6-17-55(26-39(57)52-35(41(47)60)23-40(58)59)45(64)38(19-28(2)3)54-44(63)37(22-32-24-48-27-50-32)53-43(62)36(21-29-10-8-7-9-11-29)49-25-31(16-18-65-4)51-42(61)34(46)20-30-12-14-33(56)15-13-30/h7-15,24,27-28,31,34-38,49,56H,5-6,16-23,25-26,46H2,1-4H3,(H2,47,60)(H,48,50)(H,51,61)(H,52,57)(H,53,62)(H,54,63)(H,58,59)/t31-,34+,35+,36+,37+,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAGO binding to rat brain Opioid receptor mu 1 |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031223

((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CCCCN(CC(=O)N[C@@H](CC(O)=O)C(N)=O)C(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)(C)C Show InChI InChI=1S/C45H64N10O10S/c1-6-7-18-55(25-36(57)50-33(39(47)60)23-37(58)59)44(65)38(45(2,3)4)54-43(64)35(22-29-24-48-26-49-29)53-42(63)34(21-27-11-9-8-10-12-27)52-41(62)32(17-19-66-5)51-40(61)31(46)20-28-13-15-30(56)16-14-28/h8-16,24,26,31-35,38,56H,6-7,17-23,25,46H2,1-5H3,(H2,47,60)(H,48,49)(H,50,57)(H,51,61)(H,52,62)(H,53,63)(H,54,64)(H,58,59)/t31-,32+,33-,34-,35-,38+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAGO binding to rat brain Opioid receptor mu 1 |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031221

((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CCCCN(CC(=O)N[C@@H](CC(O)=O)C(N)=O)C(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)(C)C Show InChI InChI=1S/C45H66N10O9S/c1-6-7-18-55(26-37(57)52-34(40(47)60)23-38(58)59)44(64)39(45(2,3)4)54-43(63)36(22-31-24-48-27-50-31)53-42(62)35(21-28-11-9-8-10-12-28)49-25-30(17-19-65-5)51-41(61)33(46)20-29-13-15-32(56)16-14-29/h8-16,24,27,30,33-36,39,49,56H,6-7,17-23,25-26,46H2,1-5H3,(H2,47,60)(H,48,50)(H,51,61)(H,52,57)(H,53,62)(H,54,63)(H,58,59)/t30-,33+,34+,35+,36+,39-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAGO binding to rat brain Opioid receptor mu 1 |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50009180

(3-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-pro...)Show SMILES CSCC[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(N)=O Show InChI InChI=1S/C44H62N10O10S2/c1-25(2)18-34(42(62)50-32(15-17-66-4)40(60)51-33(38(46)58)22-37(56)57)52-44(64)36(21-28-23-47-24-48-28)54-43(63)35(20-26-8-6-5-7-9-26)53-41(61)31(14-16-65-3)49-39(59)30(45)19-27-10-12-29(55)13-11-27/h5-13,23-25,30-36,55H,14-22,45H2,1-4H3,(H2,46,58)(H,47,48)(H,49,59)(H,50,62)(H,51,60)(H,52,64)(H,53,61)(H,54,63)(H,56,57)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAGO binding to rat brain Opioid receptor mu 1 |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031222

((S)-3-((S)-2-{(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Am...)Show SMILES CSCC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(=O)N[C@@H](CC(O)=O)C(N)=O)C(C)(C)C Show InChI InChI=1S/C45H64N10O10S/c1-25(2)18-33(43(64)55-37(45(3,4)5)44(65)51-32(38(47)59)22-36(57)58)52-42(63)35(21-28-23-48-24-49-28)54-41(62)34(20-26-10-8-7-9-11-26)53-40(61)31(16-17-66-6)50-39(60)30(46)19-27-12-14-29(56)15-13-27/h7-15,23-25,30-35,37,56H,16-22,46H2,1-6H3,(H2,47,59)(H,48,49)(H,50,60)(H,51,65)(H,52,63)(H,53,61)(H,54,62)(H,55,64)(H,57,58)/t30-,31+,32-,33-,34-,35-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 271 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAGO binding to rat brain Opioid receptor mu 1 |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031220

(3-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-pro...)Show SMILES CSCC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(N)=O)C(C)(C)C Show InChI InChI=1S/C44H62N10O10S2/c1-44(2,3)36(43(64)50-31(16-18-66-5)39(60)51-32(37(46)58)22-35(56)57)54-42(63)34(21-27-23-47-24-48-27)53-41(62)33(20-25-9-7-6-8-10-25)52-40(61)30(15-17-65-4)49-38(59)29(45)19-26-11-13-28(55)14-12-26/h6-14,23-24,29-34,36,55H,15-22,45H2,1-5H3,(H2,46,58)(H,47,48)(H,49,59)(H,50,64)(H,51,60)(H,52,61)(H,53,62)(H,54,63)(H,56,57)/t29-,30+,31-,32-,33-,34-,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 382 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAGO binding to rat brain Opioid receptor mu 1 |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031219

((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CC[C@H](C)N(CC(=O)N[C@@H](CC(O)=O)C(N)=O)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C45H64N10O10S/c1-6-27(4)55(24-38(57)50-34(40(47)60)22-39(58)59)45(65)37(18-26(2)3)54-44(64)36(21-30-23-48-25-49-30)53-43(63)35(20-28-10-8-7-9-11-28)52-42(62)33(16-17-66-5)51-41(61)32(46)19-29-12-14-31(56)15-13-29/h7-15,23,25-27,32-37,56H,6,16-22,24,46H2,1-5H3,(H2,47,60)(H,48,49)(H,50,57)(H,51,61)(H,52,62)(H,53,63)(H,54,64)(H,58,59)/t27-,32-,33+,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 798 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAGO binding to rat brain Opioid receptor mu 1 |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031226

((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CCCCN(CC(=O)N[C@@H](CC(O)=O)C(N)=O)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C45H64N10O10S/c1-5-6-17-55(25-38(57)50-34(40(47)60)23-39(58)59)45(65)37(19-27(2)3)54-44(64)36(22-30-24-48-26-49-30)53-43(63)35(21-28-10-8-7-9-11-28)52-42(62)33(16-18-66-4)51-41(61)32(46)20-29-12-14-31(56)15-13-29/h7-15,24,26-27,32-37,56H,5-6,16-23,25,46H2,1-4H3,(H2,47,60)(H,48,49)(H,50,57)(H,51,61)(H,52,62)(H,53,63)(H,54,64)(H,58,59)/t32-,33+,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAGO binding to rat brain Opioid receptor mu 1 |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50126171
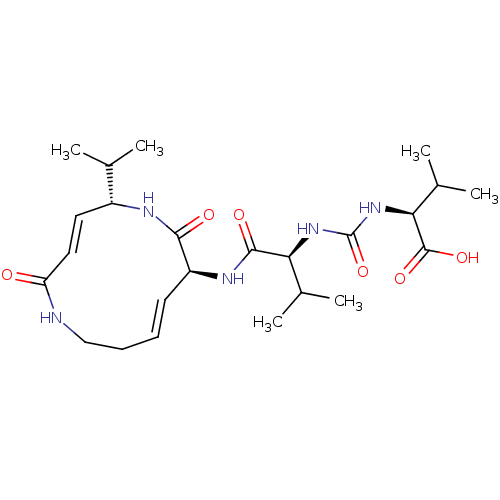
(CHEBI:80033 | CHEMBL1236043)Show SMILES CC(C)[C@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1\C=C\CCNC(=O)\C=C\[C@@H](NC1=O)C(C)C)C(O)=O |r,t:16,23| Show InChI InChI=1S/C24H39N5O6/c1-13(2)16-10-11-18(30)25-12-8-7-9-17(21(31)26-16)27-22(32)19(14(3)4)28-24(35)29-20(15(5)6)23(33)34/h7,9-11,13-17,19-20H,8,12H2,1-6H3,(H,25,30)(H,26,31)(H,27,32)(H,33,34)(H2,28,29,35)/b9-7+,11-10+/t16-,17+,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 843 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of proteasome beta-5 (unknown origin) |
Bioorg Med Chem Lett 25: 4872-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.015
BindingDB Entry DOI: 10.7270/Q2028TBR |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031219

((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CC[C@H](C)N(CC(=O)N[C@@H](CC(O)=O)C(N)=O)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C45H64N10O10S/c1-6-27(4)55(24-38(57)50-34(40(47)60)22-39(58)59)45(65)37(18-26(2)3)54-44(64)36(21-30-23-48-25-49-30)53-43(63)35(20-28-10-8-7-9-11-28)52-42(62)33(16-17-66-5)51-41(61)32(46)19-29-12-14-31(56)15-13-29/h7-15,23,25-27,32-37,56H,6,16-22,24,46H2,1-5H3,(H2,47,60)(H,48,49)(H,50,57)(H,51,61)(H,52,62)(H,53,63)(H,54,64)(H,58,59)/t27-,32-,33+,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 856 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DADLE binding to rat brain delta receptor |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031219

((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...)Show SMILES CC[C@H](C)N(CC(=O)N[C@@H](CC(O)=O)C(N)=O)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CCSC)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C45H64N10O10S/c1-6-27(4)55(24-38(57)50-34(40(47)60)22-39(58)59)45(65)37(18-26(2)3)54-44(64)36(21-30-23-48-25-49-30)53-43(63)35(20-28-10-8-7-9-11-28)52-42(62)33(16-17-66-5)51-41(61)32(46)19-29-12-14-31(56)15-13-29/h7-15,23,25-27,32-37,56H,6,16-22,24,46H2,1-5H3,(H2,47,60)(H,48,49)(H,50,57)(H,51,61)(H,52,62)(H,53,63)(H,54,64)(H,58,59)/t27-,32-,33+,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 856 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAGO binding to rat brain Opioid receptor mu 1 |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031227
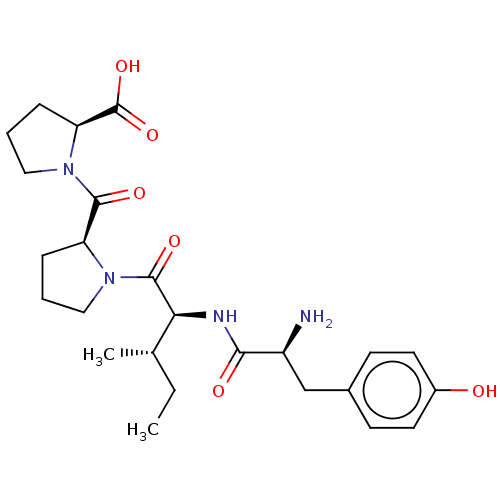
(1-(1-{(S)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-prop...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C25H36N4O6/c1-3-15(2)21(27-22(31)18(26)14-16-8-10-17(30)11-9-16)24(33)28-12-4-6-19(28)23(32)29-13-5-7-20(29)25(34)35/h8-11,15,18-21,30H,3-7,12-14,26H2,1-2H3,(H,27,31)(H,34,35)/t15-,18-,19-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DADLE binding to rat brain delta receptor |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50031227

(1-(1-{(S)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-prop...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C25H36N4O6/c1-3-15(2)21(27-22(31)18(26)14-16-8-10-17(30)11-9-16)24(33)28-12-4-6-19(28)23(32)29-13-5-7-20(29)25(34)35/h8-11,15,18-21,30H,3-7,12-14,26H2,1-2H3,(H,27,31)(H,34,35)/t15-,18-,19-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DAGO binding to rat brain Opioid receptor mu 1 |
J Med Chem 38: 3995-9 (1995)
BindingDB Entry DOI: 10.7270/Q2P849WG |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390216

(CHEMBL2070151)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1ccc(Cl)c(c1)-c1ccccc1 Show InChI InChI=1S/C21H17ClN2O2S/c1-2-27(25,26)16-9-11-19-20(13-16)24-21(23-19)15-8-10-18(22)17(12-15)14-6-4-3-5-7-14/h3-13H,2H2,1H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390219
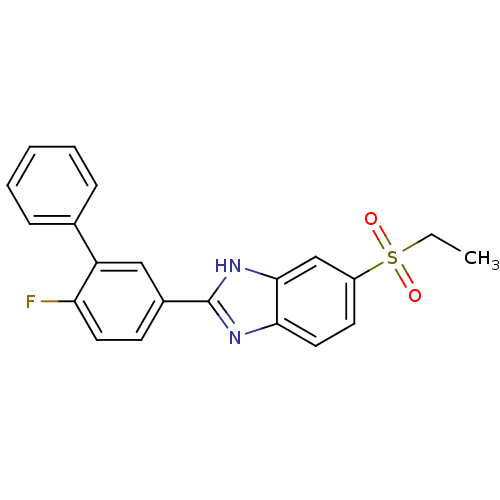
(CHEMBL2070148)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1ccc(F)c(c1)-c1ccccc1 Show InChI InChI=1S/C21H17FN2O2S/c1-2-27(25,26)16-9-11-19-20(13-16)24-21(23-19)15-8-10-18(22)17(12-15)14-6-4-3-5-7-14/h3-13H,2H2,1H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390213
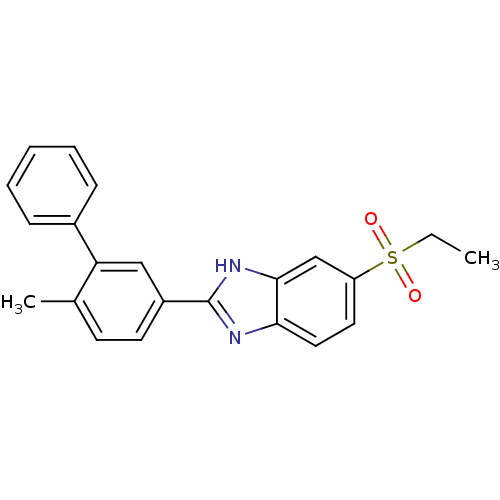
(CHEMBL2069316)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1ccc(C)c(c1)-c1ccccc1 Show InChI InChI=1S/C22H20N2O2S/c1-3-27(25,26)18-11-12-20-21(14-18)24-22(23-20)17-10-9-15(2)19(13-17)16-7-5-4-6-8-16/h4-14H,3H2,1-2H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394224
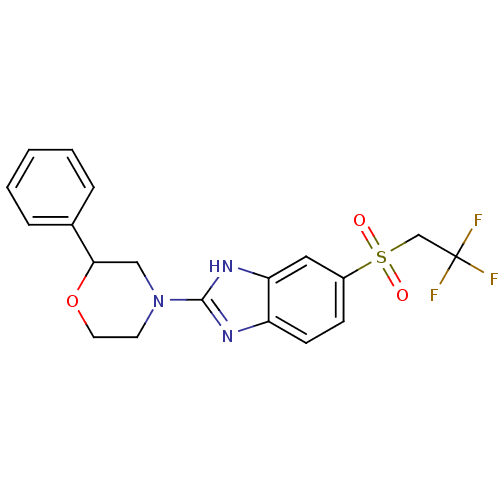
(CHEMBL2159167)Show SMILES FC(F)(F)CS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C19H18F3N3O3S/c20-19(21,22)12-29(26,27)14-6-7-15-16(10-14)24-18(23-15)25-8-9-28-17(11-25)13-4-2-1-3-5-13/h1-7,10,17H,8-9,11-12H2,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394211
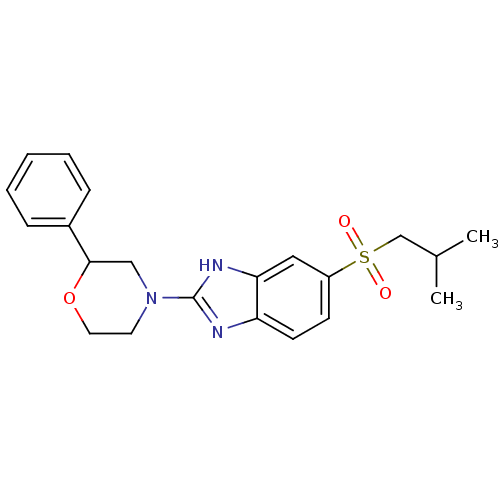
(CHEMBL2159165)Show SMILES CC(C)CS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C21H25N3O3S/c1-15(2)14-28(25,26)17-8-9-18-19(12-17)23-21(22-18)24-10-11-27-20(13-24)16-6-4-3-5-7-16/h3-9,12,15,20H,10-11,13-14H2,1-2H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394221
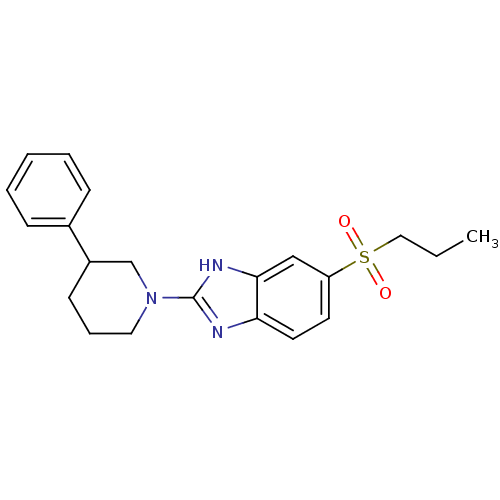
(CHEMBL2159158)Show SMILES CCCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCCC(C1)c1ccccc1 Show InChI InChI=1S/C21H25N3O2S/c1-2-13-27(25,26)18-10-11-19-20(14-18)23-21(22-19)24-12-6-9-17(15-24)16-7-4-3-5-8-16/h3-5,7-8,10-11,14,17H,2,6,9,12-13,15H2,1H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
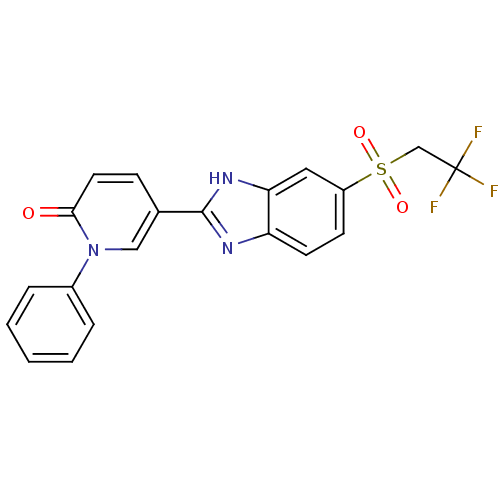
(Mus musculus (Mouse)) | BDBM50390228
(CHEMBL2070161)Show SMILES FC(F)(F)CS(=O)(=O)c1ccc2nc([nH]c2c1)-c1ccc(=O)n(c1)-c1ccccc1 Show InChI InChI=1S/C20H14F3N3O3S/c21-20(22,23)12-30(28,29)15-7-8-16-17(10-15)25-19(24-16)13-6-9-18(27)26(11-13)14-4-2-1-3-5-14/h1-11H,12H2,(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
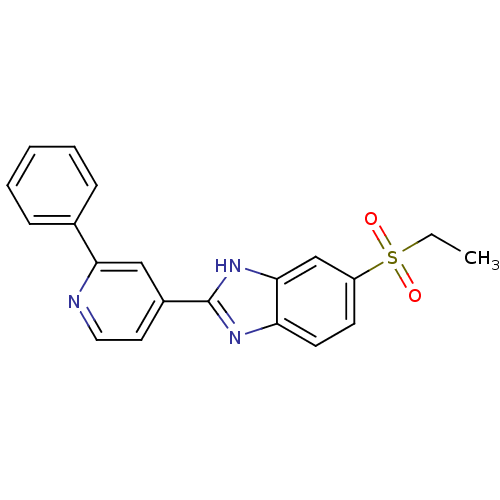
(Mus musculus (Mouse)) | BDBM50390232
(CHEMBL2070157)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1ccnc(c1)-c1ccccc1 Show InChI InChI=1S/C20H17N3O2S/c1-2-26(24,25)16-8-9-17-19(13-16)23-20(22-17)15-10-11-21-18(12-15)14-6-4-3-5-7-14/h3-13H,2H2,1H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394214

(CHEMBL2159162)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C19H21N3O3S/c1-2-26(23,24)15-8-9-16-17(12-15)21-19(20-16)22-10-11-25-18(13-22)14-6-4-3-5-7-14/h3-9,12,18H,2,10-11,13H2,1H3,(H,20,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390225

(CHEMBL2070140)Show SMILES CCCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C22H20N2O2S/c1-2-13-27(25,26)19-11-12-20-21(15-19)24-22(23-20)18-10-6-9-17(14-18)16-7-4-3-5-8-16/h3-12,14-15H,2,13H2,1H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394214

(CHEMBL2159162)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C19H21N3O3S/c1-2-26(23,24)15-8-9-16-17(12-15)21-19(20-16)22-10-11-25-18(13-22)14-6-4-3-5-7-14/h3-9,12,18H,2,10-11,13H2,1H3,(H,20,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY-Y5 receptor |
Bioorg Med Chem Lett 23: 90-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.11.005
BindingDB Entry DOI: 10.7270/Q2FB547S |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390222
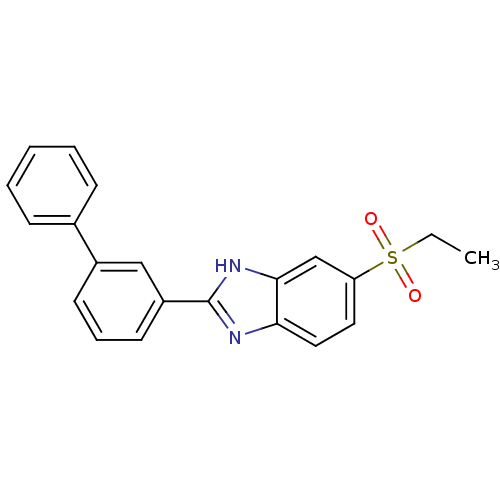
(CHEMBL2070137)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C21H18N2O2S/c1-2-26(24,25)18-11-12-19-20(14-18)23-21(22-19)17-10-6-9-16(13-17)15-7-4-3-5-8-15/h3-14H,2H2,1H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390220
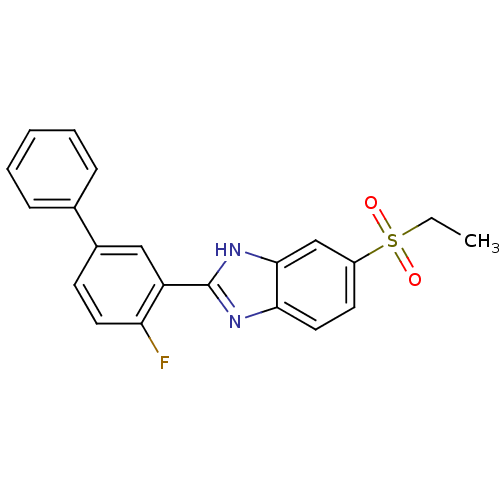
(CHEMBL2070147)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1cc(ccc1F)-c1ccccc1 Show InChI InChI=1S/C21H17FN2O2S/c1-2-27(25,26)16-9-11-19-20(13-16)24-21(23-19)17-12-15(8-10-18(17)22)14-6-4-3-5-7-14/h3-13H,2H2,1H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50390234

(CHEMBL2070155)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1cc(ccn1)-c1ccccc1 Show InChI InChI=1S/C20H17N3O2S/c1-2-26(24,25)16-8-9-17-18(13-16)23-20(22-17)19-12-15(10-11-21-19)14-6-4-3-5-7-14/h3-13H,2H2,1H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394218

(CHEMBL2159156)Show SMILES CCCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C20H23N3O3S/c1-2-12-27(24,25)16-8-9-17-18(13-16)22-20(21-17)23-10-11-26-19(14-23)15-6-4-3-5-7-15/h3-9,13,19H,2,10-12,14H2,1H3,(H,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394223
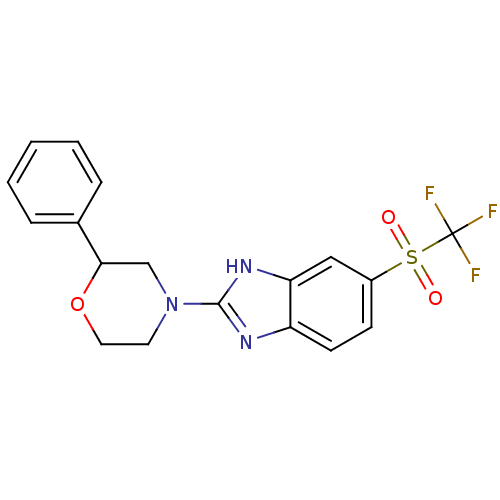
(CHEMBL2159166)Show SMILES FC(F)(F)S(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C18H16F3N3O3S/c19-18(20,21)28(25,26)13-6-7-14-15(10-13)23-17(22-14)24-8-9-27-16(11-24)12-4-2-1-3-5-12/h1-7,10,16H,8-9,11H2,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394228

(CHEMBL2159171)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccc(C)cc1 Show InChI InChI=1S/C20H23N3O3S/c1-3-27(24,25)16-8-9-17-18(12-16)22-20(21-17)23-10-11-26-19(13-23)15-6-4-14(2)5-7-15/h4-9,12,19H,3,10-11,13H2,1-2H3,(H,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394227
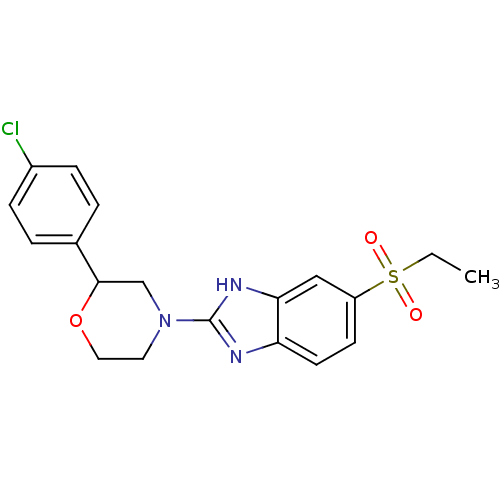
(CHEMBL2159170)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccc(Cl)cc1 Show InChI InChI=1S/C19H20ClN3O3S/c1-2-27(24,25)15-7-8-16-17(11-15)22-19(21-16)23-9-10-26-18(12-23)13-3-5-14(20)6-4-13/h3-8,11,18H,2,9-10,12H2,1H3,(H,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
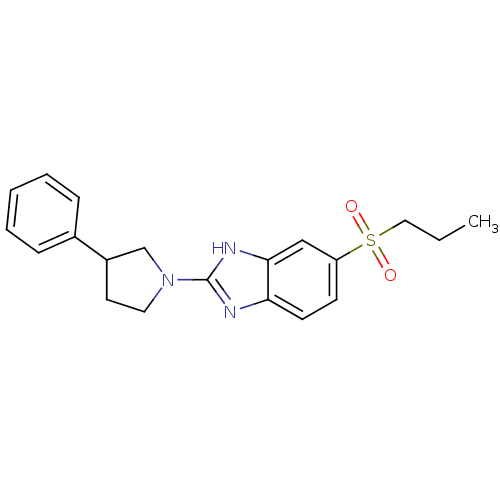
(Mus musculus (Mouse)) | BDBM50394222
(CHEMBL2159174)Show SMILES CCCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCC(C1)c1ccccc1 Show InChI InChI=1S/C20H23N3O2S/c1-2-12-26(24,25)17-8-9-18-19(13-17)22-20(21-18)23-11-10-16(14-23)15-6-4-3-5-7-15/h3-9,13,16H,2,10-12,14H2,1H3,(H,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
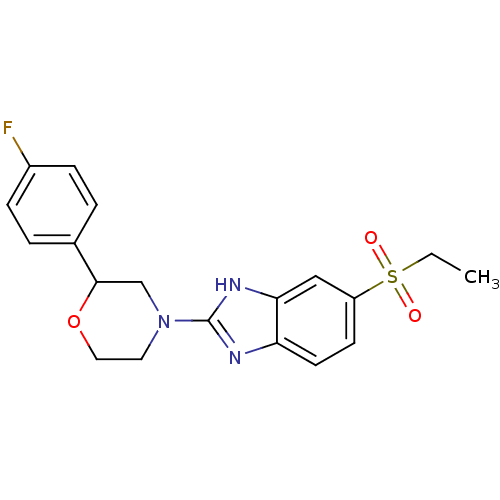
(Mus musculus (Mouse)) | BDBM50394226
(CHEMBL2159169)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccc(F)cc1 Show InChI InChI=1S/C19H20FN3O3S/c1-2-27(24,25)15-7-8-16-17(11-15)22-19(21-16)23-9-10-26-18(12-23)13-3-5-14(20)6-4-13/h3-8,11,18H,2,9-10,12H2,1H3,(H,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394213

(CHEMBL2159163)Show SMILES CC(C)S(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C20H23N3O3S/c1-14(2)27(24,25)16-8-9-17-18(12-16)22-20(21-17)23-10-11-26-19(13-23)15-6-4-3-5-7-15/h3-9,12,14,19H,10-11,13H2,1-2H3,(H,21,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.33 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Mus musculus (Mouse)) | BDBM50394225

(CHEMBL2159168)Show SMILES CC(C)(C(F)(F)F)S(=O)(=O)c1ccc2nc([nH]c2c1)N1CCOC(C1)c1ccccc1 Show InChI InChI=1S/C21H22F3N3O3S/c1-20(2,21(22,23)24)31(28,29)15-8-9-16-17(12-15)26-19(25-16)27-10-11-30-18(13-27)14-6-4-3-5-7-14/h3-9,12,18H,10-11,13H2,1-2H3,(H,25,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 6554-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.025
BindingDB Entry DOI: 10.7270/Q21V5G3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
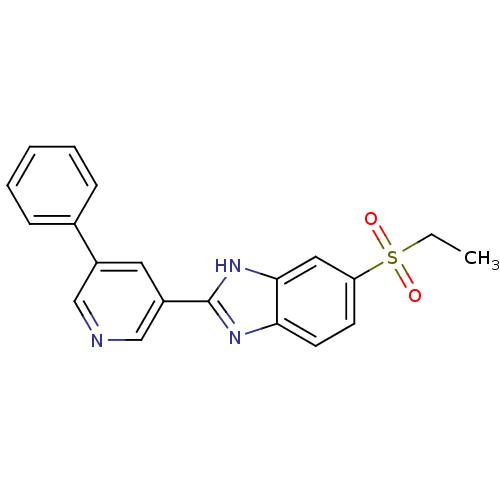
(Mus musculus (Mouse)) | BDBM50390233
(CHEMBL2070156)Show SMILES CCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1cncc(c1)-c1ccccc1 Show InChI InChI=1S/C20H17N3O2S/c1-2-26(24,25)17-8-9-18-19(11-17)23-20(22-18)16-10-15(12-21-13-16)14-6-4-3-5-7-14/h3-13H,2H2,1H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
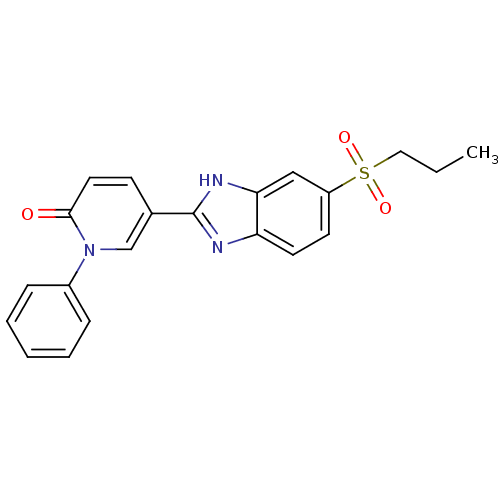
(Mus musculus (Mouse)) | BDBM50390231
(CHEMBL2070158)Show SMILES CCCS(=O)(=O)c1ccc2nc([nH]c2c1)-c1ccc(=O)n(c1)-c1ccccc1 Show InChI InChI=1S/C21H19N3O3S/c1-2-12-28(26,27)17-9-10-18-19(13-17)23-21(22-18)15-8-11-20(25)24(14-15)16-6-4-3-5-7-16/h3-11,13-14H,2,12H2,1H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]PYY from mouse NPY Y5 receptor |
Bioorg Med Chem Lett 22: 5498-502 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.020
BindingDB Entry DOI: 10.7270/Q2251K8Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data