

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50001935 (CHEMBL3233601) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50001936 (CHEMBL3233602) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50001934 (CHEMBL3233600) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50001936 (CHEMBL3233602) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50001933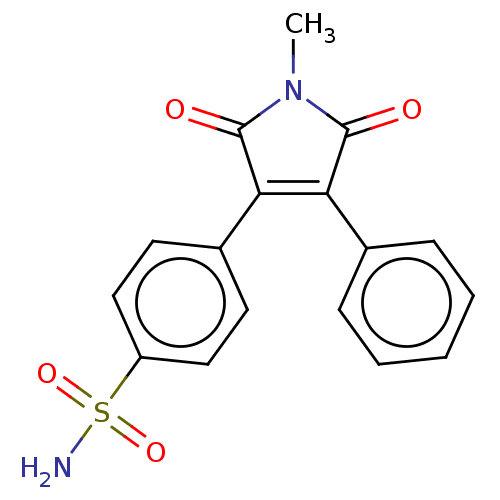 (CHEMBL3233599) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50001933 (CHEMBL3233599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50001935 (CHEMBL3233601) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50001934 (CHEMBL3233600) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50222222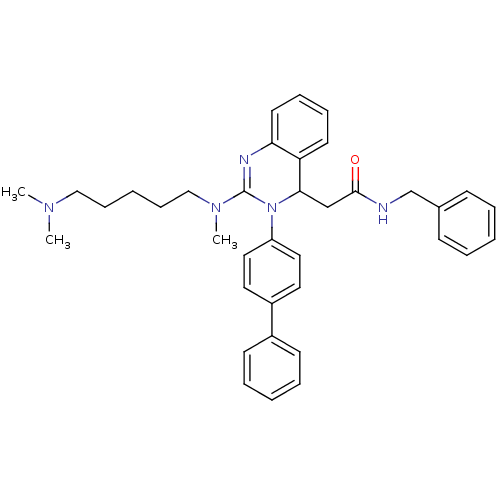 (CHEMBL394956 | KYS-05090 | N-benzyl-2-(3-(biphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type CaV3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of calcium current by whole cell patch-clamp me... | Bioorg Med Chem Lett 24: 1565-70 (2014) Article DOI: 10.1016/j.bmcl.2014.01.071 BindingDB Entry DOI: 10.7270/Q2MK6FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50222222 (CHEMBL394956 | KYS-05090 | N-benzyl-2-(3-(biphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50494713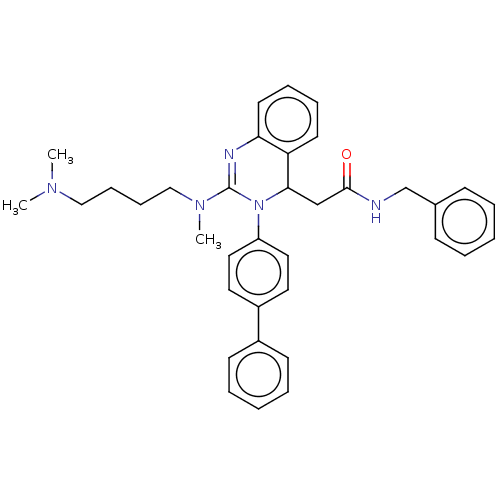 (CHEMBL3093776) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50232642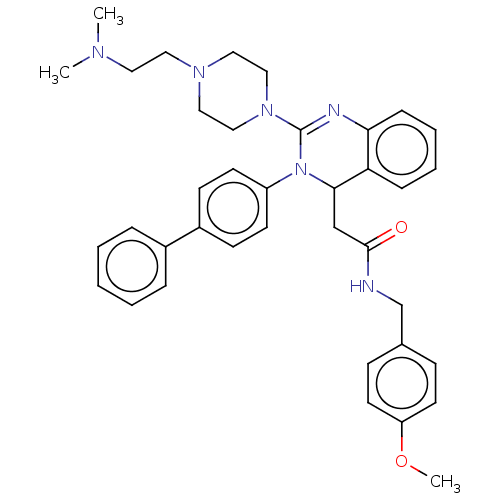 (CHEMBL3093781) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50232643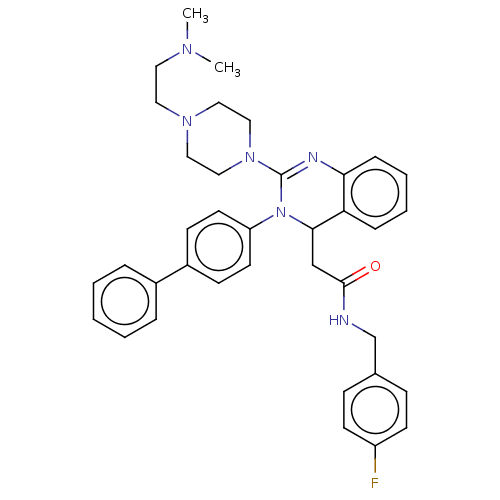 (CHEMBL3093780) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50494712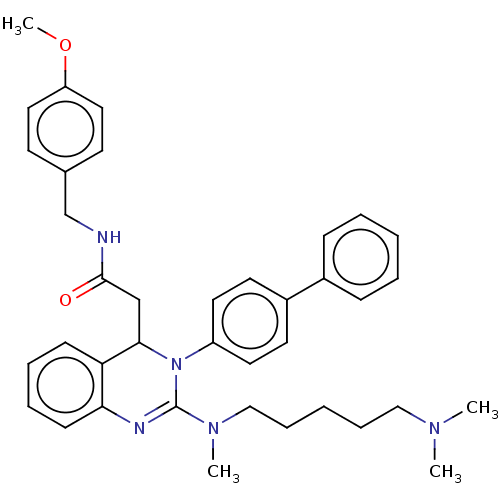 (CHEMBL3093774) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50001931 (CHEMBL3233597) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50494711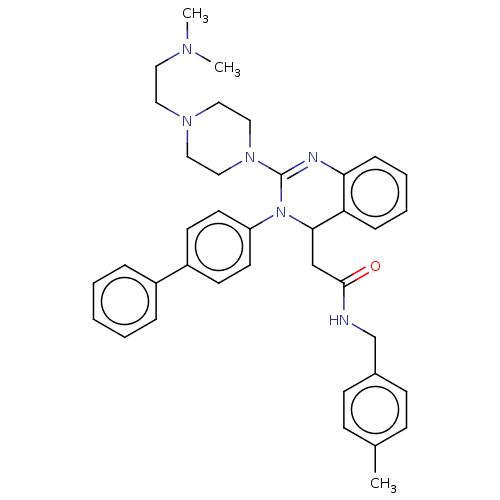 (CHEMBL3093779) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50007615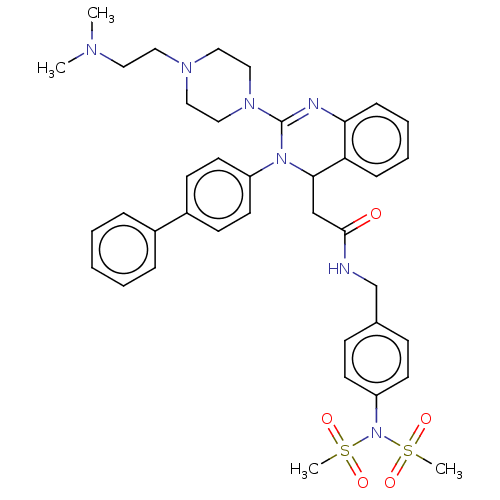 (CHEMBL3233080) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type CaV3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of calcium current by whole cell patch-clamp me... | Bioorg Med Chem Lett 24: 1565-70 (2014) Article DOI: 10.1016/j.bmcl.2014.01.071 BindingDB Entry DOI: 10.7270/Q2MK6FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50494710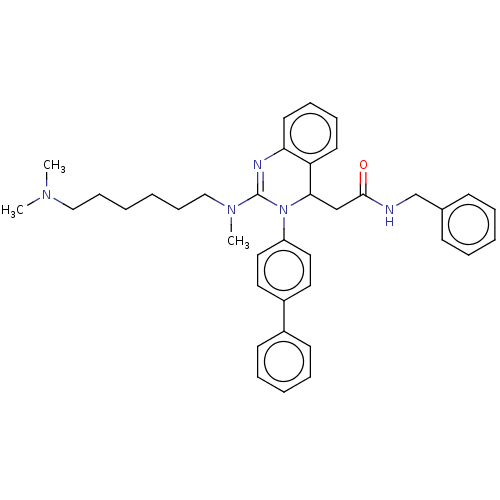 (CHEMBL3093777) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50117922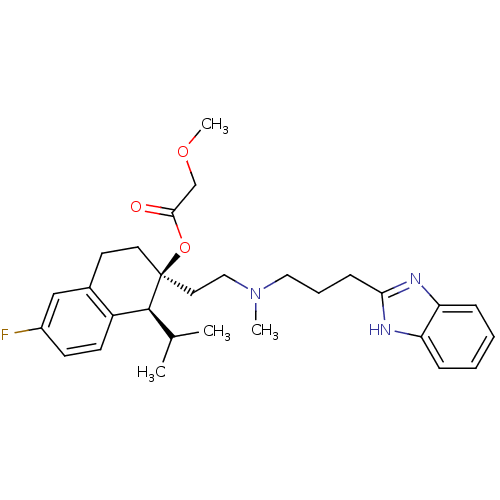 ((1S,2S)-2-(2-((3-(1H-benzo[d]imidazol-2-yl)propyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type CaV3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of calcium current by whole cell patch-clamp me... | Bioorg Med Chem Lett 24: 1565-70 (2014) Article DOI: 10.1016/j.bmcl.2014.01.071 BindingDB Entry DOI: 10.7270/Q2MK6FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50117922 ((1S,2S)-2-(2-((3-(1H-benzo[d]imidazol-2-yl)propyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50007613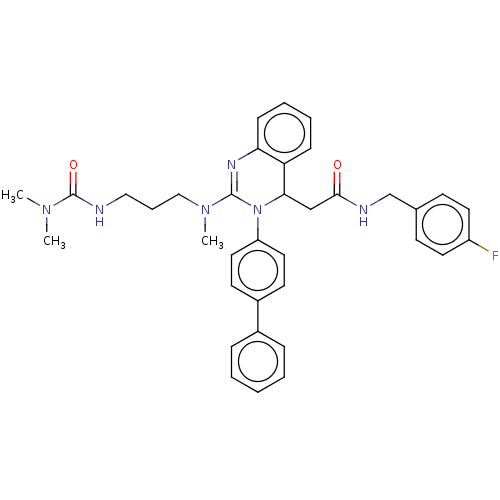 (CHEMBL3233076) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type CaV3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of calcium current by whole cell patch-clamp me... | Bioorg Med Chem Lett 24: 1565-70 (2014) Article DOI: 10.1016/j.bmcl.2014.01.071 BindingDB Entry DOI: 10.7270/Q2MK6FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50305810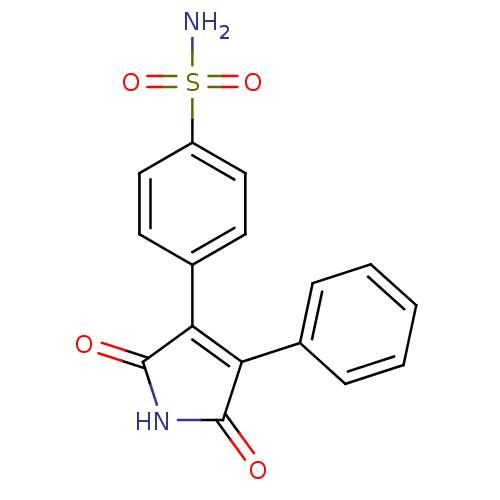 (1H-3-(4-sulfamoylphenyl)-4-phenyl-pyrrole-2,5-dion...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50007612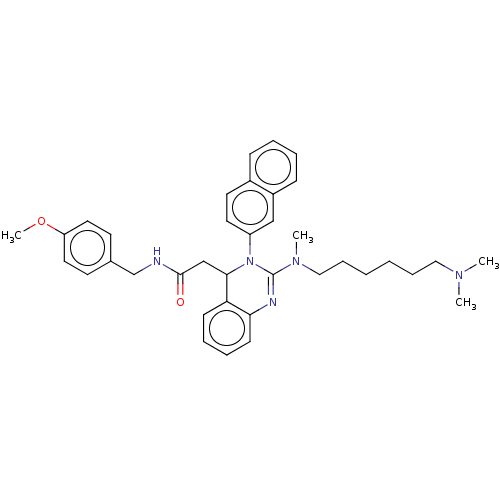 (CHEMBL3233075) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type CaV3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of calcium current by whole cell patch-clamp me... | Bioorg Med Chem Lett 24: 1565-70 (2014) Article DOI: 10.1016/j.bmcl.2014.01.071 BindingDB Entry DOI: 10.7270/Q2MK6FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50009859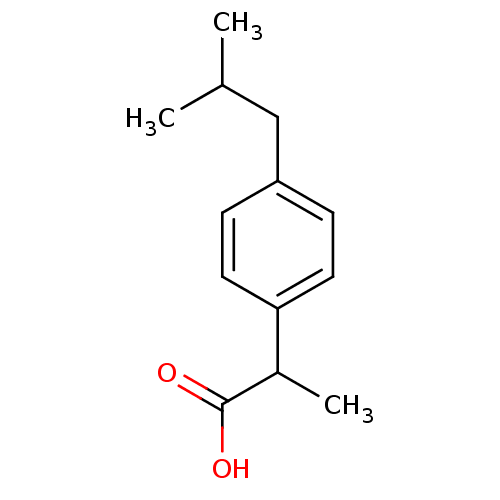 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50001930 (CHEMBL3233596) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50001933 (CHEMBL3233599) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50001934 (CHEMBL3233600) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50001935 (CHEMBL3233601) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50001936 (CHEMBL3233602) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50007614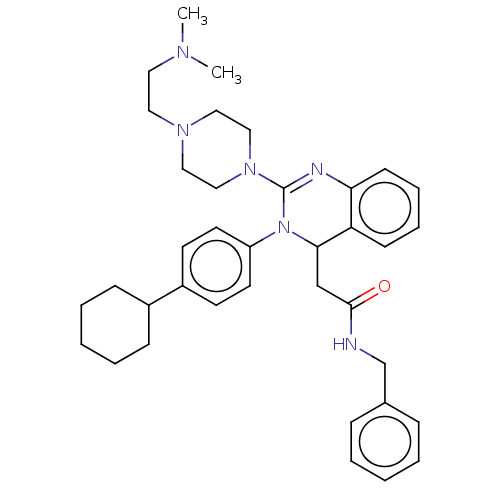 (CHEMBL3233078) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type CaV3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of calcium current by whole cell patch-clamp me... | Bioorg Med Chem Lett 24: 1565-70 (2014) Article DOI: 10.1016/j.bmcl.2014.01.071 BindingDB Entry DOI: 10.7270/Q2MK6FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50001931 (CHEMBL3233597) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50001930 (CHEMBL3233596) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50007611 (CHEMBL3233073) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type CaV3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of calcium current by whole cell patch-clamp me... | Bioorg Med Chem Lett 24: 1565-70 (2014) Article DOI: 10.1016/j.bmcl.2014.01.071 BindingDB Entry DOI: 10.7270/Q2MK6FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50001932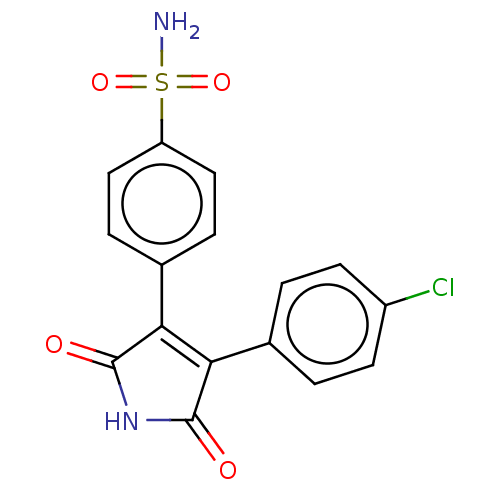 (CHEMBL3233598) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50305810 (1H-3-(4-sulfamoylphenyl)-4-phenyl-pyrrole-2,5-dion...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50009859 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50001932 (CHEMBL3233598) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50009859 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50001932 (CHEMBL3233598) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50001931 (CHEMBL3233597) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50001930 (CHEMBL3233596) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50305810 (1H-3-(4-sulfamoylphenyl)-4-phenyl-pyrrole-2,5-dion...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||