 Found 483 hits with Last Name = 'christensen' and Initial = 'sb'
Found 483 hits with Last Name = 'christensen' and Initial = 'sb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606633
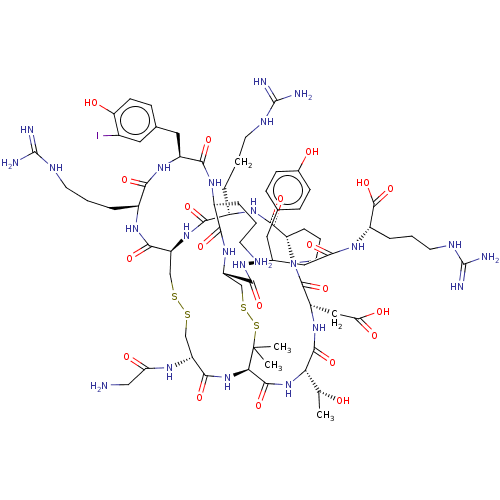
(CHEMBL5219936)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CSSC1(C)C)C(=O)N[C@H](CC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)Cc1ccc(O)cc1)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50035612
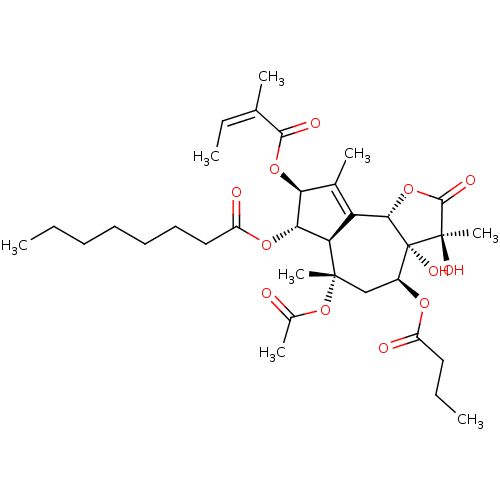
(CHEMBL96926 | OCTANOIC ACID [3S-[3ALPHA, 3ABETA, 4...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)C(\C)=C/C)C(C)=C2[C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@](C)(OC(C)=O)[C@@H]12)OC(=O)CCC |r,t:20| Show InChI InChI=1S/C34H50O12/c1-9-12-13-14-15-17-24(37)43-28-26-25(20(5)27(28)44-30(38)19(4)11-3)29-34(41,33(8,40)31(39)45-29)22(42-23(36)16-10-2)18-32(26,7)46-21(6)35/h11,22,26-29,40-41H,9-10,12-18H2,1-8H3/b19-11-/t22-,26+,27-,28-,29-,32-,33+,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606639
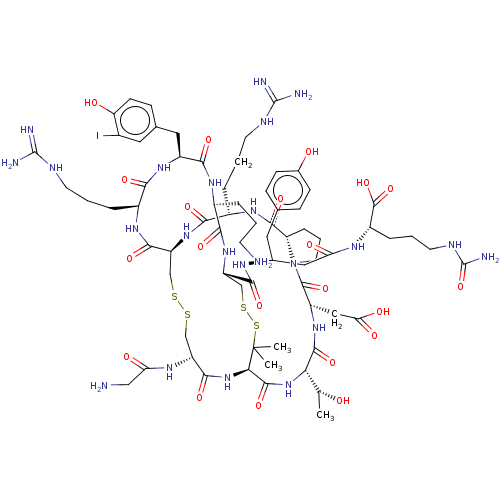
(CHEMBL5219016)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CSSC1(C)C)C(=O)N[C@H](CC(=O)N[C@@H](CCCNC(N)=O)C(O)=O)Cc1ccc(O)cc1)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606640
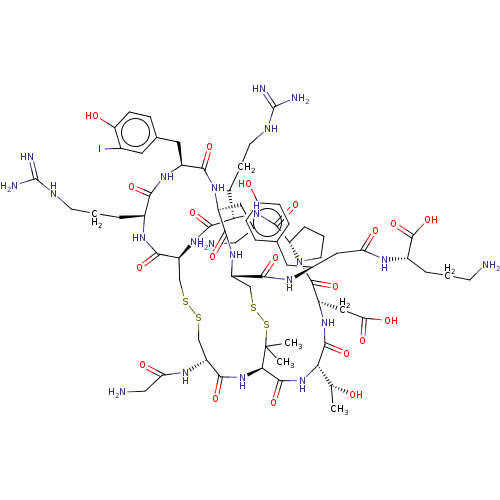
(CHEMBL5220467)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CSSC1(C)C)C(=O)N[C@H](CC(=O)N[C@@H](CCCN)C(O)=O)Cc1ccc(O)cc1)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606641
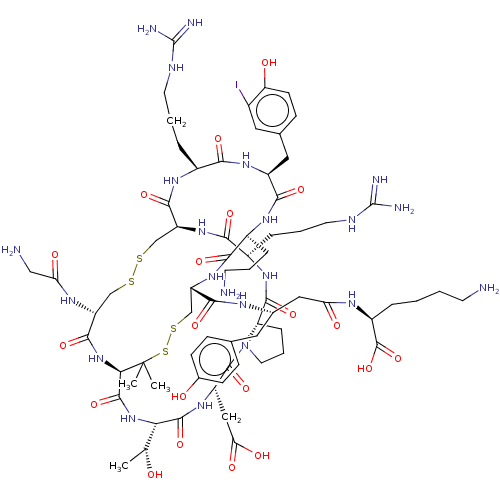
(CHEMBL5219752)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CSSC1(C)C)C(=O)N[C@H](CC(=O)N[C@@H](CCCCN)C(O)=O)Cc1ccc(O)cc1)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor alpha9/alpha10
(RAT-Rattus norvegicus) | BDBM50606633

(CHEMBL5219936)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CSSC1(C)C)C(=O)N[C@H](CC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)Cc1ccc(O)cc1)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606645

(CHEMBL5220455)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CSSC1(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606638
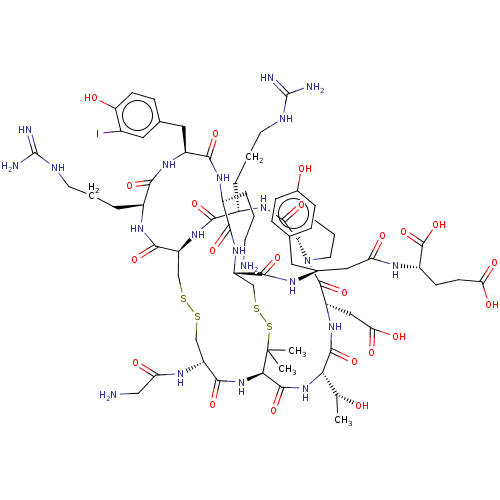
(CHEMBL5220113)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CSSC1(C)C)C(=O)N[C@H](CC(=O)N[C@@H](CCC(O)=O)C(O)=O)Cc1ccc(O)cc1)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50581244

(CHEMBL5086734)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc3ccc(O)c(I)c3)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CSSC[C@H](NC(=O)CN)C(=O)N2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(O)=O)NC1=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human alpha9alpha10 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced current response by two-e... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00802
BindingDB Entry DOI: 10.7270/Q2542SG3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606644

(CHEMBL5219791)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CSSC1(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326748

((3S,3aR,4S,6S,6aR,7S,8S,9bS)-6-acetoxy-4-(butyrylo...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)c2ccccc2)C(C)=C2[C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@](C)(OC(C)=O)[C@@H]12)OC(=O)CCC |r,t:23| Show InChI InChI=1S/C36H48O12/c1-7-9-10-11-15-19-26(39)45-30-28-27(21(3)29(30)46-32(40)23-17-13-12-14-18-23)31-36(43,35(6,42)33(41)47-31)24(44-25(38)16-8-2)20-34(28,5)48-22(4)37/h12-14,17-18,24,28-31,42-43H,7-11,15-16,19-20H2,1-6H3/t24-,28+,29-,30-,31-,34-,35+,36+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50581248

(CHEMBL5090753)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@@H]2NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]3CCCN3C(=O)[C@H](CC(O)O)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)c(I)c1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CSCS2)C(=O)N[C@H](CC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)Cc1ccc(O)cc1)NC(=O)CN |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human alpha9alpha10 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced current response by two-e... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00802
BindingDB Entry DOI: 10.7270/Q2542SG3 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor alpha9/alpha10
(RAT-Rattus norvegicus) | BDBM50606635

(CHEMBL5220820)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1C\C=C\C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@H](CSSC[C@H](NC(=O)CN)C(=O)N1)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r,t:25| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Homo sapiens (Human)) | BDBM50165369

(12-(4-Amino-4-carboxy-butyrylamino)-dodecanoic aci...)Show SMILES CCCCCCCC(=O)O[C@H]1[C@H]2C([C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@]2(C)OC(C)=O)OC(=O)CCCCCCCCCCCNC(=O)CCC(N)C(O)=O)=C(C)C1=O |t:55| Show InChI InChI=1S/C42H66N2O14/c1-6-7-8-14-17-21-32(48)56-36-34-33(26(2)35(36)49)37-42(54,41(5,53)39(52)57-37)29(25-40(34,4)58-27(3)45)55-31(47)20-18-15-12-10-9-11-13-16-19-24-44-30(46)23-22-28(43)38(50)51/h28-29,34,36-37,53-54H,6-25,43H2,1-5H3,(H,44,46)(H,50,51)/t28?,29-,34+,36-,37-,40-,41+,42+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibitory activity against sarco- endoplasmic reticulum calcium-ATPase |
J Med Chem 48: 3005-14 (2005)
Article DOI: 10.1021/jm049319a
BindingDB Entry DOI: 10.7270/Q27W6BP4 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606650
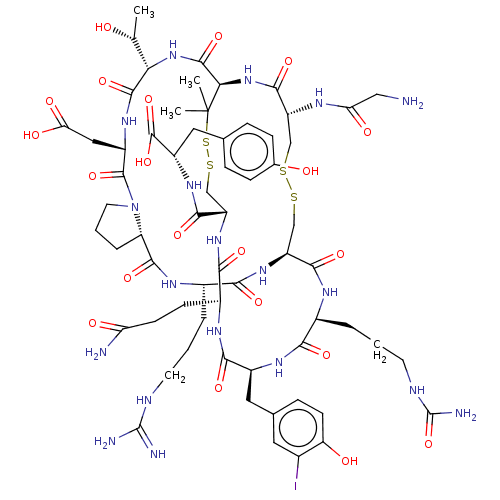
(CHEMBL5219058)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=O)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CSSC1(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606637
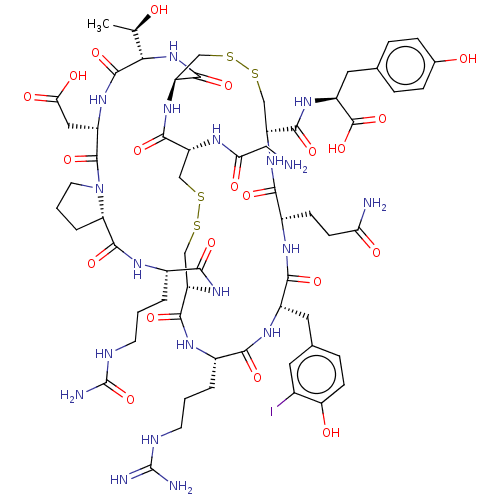
(CHEMBL5220002)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc3ccc(O)c(I)c3)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CSSC[C@@H](NC(=O)CN)C(=O)N1)NC(=O)[C@H](CCCNC(N)=O)NC2=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor alpha9/alpha10
(RAT-Rattus norvegicus) | BDBM50606636
(CHEMBL5220778)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@H](CSSC[C@H](NC(=O)CN)C(=O)N1)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50544003
(CHEMBL4647678)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=O)C(=O)N[C@@H](Cc1ccc(O)c(I)c1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C62H92IN19O21S4/c1-28(83)48(81-56(97)42(27-107)80-53(94)39(24-104)71-46(87)23-64)58(99)76-37(22-47(88)89)59(100)82-18-4-7-43(82)57(98)74-34(5-2-16-69-61(66)67)50(91)78-40(25-105)54(95)72-33(6-3-17-70-62(68)103)49(90)75-36(21-30-10-14-44(85)32(63)19-30)52(93)73-35(13-15-45(65)86)51(92)79-41(26-106)55(96)77-38(60(101)102)20-29-8-11-31(84)12-9-29/h8-12,14,19,28,33-43,48,83-85,104-107H,2-7,13,15-18,20-27,64H2,1H3,(H2,65,86)(H,71,87)(H,72,95)(H,73,93)(H,74,98)(H,75,90)(H,76,99)(H,77,96)(H,78,91)(H,79,92)(H,80,94)(H,81,97)(H,88,89)(H,101,102)(H4,66,67,69)(H3,68,70,103)/t28-,33+,34+,35+,36+,37+,38+,39+,40+,41+,42+,43+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha9alpha10 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of Ach-induced response measured after 5 ... |
J Med Chem 63: 8380-8387 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00613
BindingDB Entry DOI: 10.7270/Q2474FDJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50581243
(CHEMBL5089881)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]3CCCN3C(=O)[C@H](CC(O)=O)NC1=O)C(=O)N[C@@H](CCCNC(N)=O)C(=O)N[C@@H](Cc1ccc(O)c(I)c1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CSSC[C@H](NC(=O)CN)C(=O)N2)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human alpha9alpha10 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced current response by two-e... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00802
BindingDB Entry DOI: 10.7270/Q2542SG3 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor alpha9/alpha10
(RAT-Rattus norvegicus) | BDBM50606634

(CHEMBL5220229)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1C\C=C/C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@H](CSSC[C@H](NC(=O)CN)C(=O)N1)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r,c:25| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326743
((3S,3aR,4S,6S,6aR,7S,8S,9bS)-6-acetoxy-4-(butyrylo...)Show SMILES CCCC(=O)O[C@H]1C[C@](C)(OC(C)=O)[C@H]2[C@H](OC(=O)c3ccccc3)[C@@H](OC(=O)C(\C)=C/C)C(C)=C2[C@@H]2OC(=O)[C@@](C)(O)[C@@]12O |r,c:35| Show InChI InChI=1S/C33H40O12/c1-8-13-22(35)41-21-16-31(6,45-19(5)34)24-23(27-33(21,40)32(7,39)30(38)44-27)18(4)25(42-28(36)17(3)9-2)26(24)43-29(37)20-14-11-10-12-15-20/h9-12,14-15,21,24-27,39-40H,8,13,16H2,1-7H3/b17-9-/t21-,24+,25-,26-,27-,31-,32+,33+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Homo sapiens (Human)) | BDBM50165368

(CHEMBL191633 | Dodecanedioic acid mono-((2S,3aR,4S...)Show SMILES CCCCCCCC(=O)O[C@H]1[C@H]2C([C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@]2(C)OC(C)=O)OC(=O)CCCCCCCCCCC(O)=O)=C(C)C1=O |t:47| Show InChI InChI=1S/C37H56O13/c1-6-7-8-13-17-21-28(42)48-32-30-29(23(2)31(32)43)33-37(46,36(5,45)34(44)49-33)25(22-35(30,4)50-24(3)38)47-27(41)20-18-15-12-10-9-11-14-16-19-26(39)40/h25,30,32-33,45-46H,6-22H2,1-5H3,(H,39,40)/t25-,30+,32-,33-,35-,36+,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibitory activity against sarco- endoplasmic reticulum calcium-ATPase |
J Med Chem 48: 3005-14 (2005)
Article DOI: 10.1021/jm049319a
BindingDB Entry DOI: 10.7270/Q27W6BP4 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606643
(CHEMBL5218653)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CSSC1(C)C)C(=O)N[C@H](Cc1ccc(O)cc1)C(=O)N[C@H](CCCNC(N)=N)C(O)=O)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326737

((3S,3aR,4S,6S,6aR,7S,8S,9bS)-3,3a-dihydroxy-3,6,9-...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)C(\C)=C/C)C(C)=C2[C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@](C)(OC(=O)CCC)[C@@H]12)OC(=O)CCC |r,t:20| Show InChI InChI=1S/C36H54O12/c1-9-13-14-15-16-19-25(38)45-30-28-27(22(6)29(30)46-32(40)21(5)12-4)31-36(43,35(8,42)33(41)47-31)23(44-24(37)17-10-2)20-34(28,7)48-26(39)18-11-3/h12,23,28-31,42-43H,9-11,13-20H2,1-8H3/b21-12-/t23-,28+,29-,30-,31-,34-,35+,36+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50581249
(CHEMBL5073182)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@@H]2NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]3CCCN3C(=O)[C@H](CC(O)O)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)c(I)c1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CSCS2)C(=O)NCCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)NC(=O)CN |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human alpha9alpha10 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced current response by two-e... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00802
BindingDB Entry DOI: 10.7270/Q2542SG3 |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326751
((3S,3aR,4S,6S,6aR,7S,8S,9bS)-6-acetoxy-4-(butyrylo...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)c2ccccc2-c2ccccc2)C(C)=C2[C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@](C)(OC(C)=O)[C@@H]12)OC(=O)CCC |r,t:30| Show InChI InChI=1S/C42H52O12/c1-7-9-10-11-15-23-32(45)51-36-34-33(25(3)35(36)52-38(46)29-22-17-16-21-28(29)27-19-13-12-14-20-27)37-42(49,41(6,48)39(47)53-37)30(50-31(44)18-8-2)24-40(34,5)54-26(4)43/h12-14,16-17,19-22,30,34-37,48-49H,7-11,15,18,23-24H2,1-6H3/t30-,34+,35-,36-,37-,40-,41+,42+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50544009
(CHEMBL4644407)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H]2CSSC[C@@H]3NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc4ccc(O)c(I)c4)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)[C@@H](N)CCC(=O)NCCCC[C@@H](NC(=O)[C@H](Cc4ccc(O)cc4)NC3=O)C(O)=O)C(=O)N2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(O)=O)NC1=O |r| Show InChI InChI=1S/C73H106IN23O23S4/c1-34(98)57-69(117)92-46(28-56(104)105)70(118)97-24-6-10-51(97)68(116)88-41(9-5-23-83-73(79)80)60(108)93-48-31-122-121-30-47-64(112)95-50(67(115)96-57)33-124-123-32-49(94-61(109)42(17-19-53(76)101)87-62(110)45(27-36-13-18-52(100)38(74)25-36)90-59(107)40(86-65(48)113)8-4-22-82-72(77)78)66(114)91-44(26-35-11-14-37(99)15-12-35)63(111)89-43(71(119)120)7-2-3-21-81-54(102)20-16-39(75)58(106)84-29-55(103)85-47/h11-15,18,25,34,39-51,57,98-100H,2-10,16-17,19-24,26-33,75H2,1H3,(H2,76,101)(H,81,102)(H,84,106)(H,85,103)(H,86,113)(H,87,110)(H,88,116)(H,89,111)(H,90,107)(H,91,114)(H,92,117)(H,93,108)(H,94,109)(H,95,112)(H,96,115)(H,104,105)(H,119,120)(H4,77,78,82)(H4,79,80,83)/t34-,39+,40+,41+,42+,43-,44+,45+,46+,47+,48+,49+,50+,51+,57+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha9alpha10 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of Ach-induced response measured after 5 ... |
J Med Chem 63: 8380-8387 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00613
BindingDB Entry DOI: 10.7270/Q2474FDJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606642
(CHEMBL5220060)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CSSC1(C)C)C(=O)N[C@H](CC(=O)N[C@H](CCCNC(N)=N)C(O)=O)Cc1ccc(O)cc1)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50042058

((-)-rolipram | (4R)-4-[3-(cyclopentyloxy)-4-methox...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol |
J Med Chem 41: 821-35 (1998)
Article DOI: 10.1021/jm970090r
BindingDB Entry DOI: 10.7270/Q2WD439M |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50472190

(CHEMBL167638)Show InChI InChI=1S/C18H24O4/c1-21-16-9-8-13(12-6-7-14(10-12)18(19)20)11-17(16)22-15-4-2-3-5-15/h8-9,11-12,14-15H,2-7,10H2,1H3,(H,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol |
J Med Chem 41: 821-35 (1998)
Article DOI: 10.1021/jm970090r
BindingDB Entry DOI: 10.7270/Q2WD439M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50544008
(CHEMBL4634686)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H]2CSSC[C@@H]3NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc4ccc(O)c(I)c4)NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)[C@@H](N)CCC(=O)NCCCC[C@@H](NC(=O)[C@H](Cc4ccc(O)cc4)NC3=O)C(O)=O)C(=O)N2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(O)=O)NC1=O |r| Show InChI InChI=1S/C73H105IN22O24S4/c1-34(97)57-69(116)91-46(28-56(103)104)70(117)96-24-6-10-51(96)68(115)87-41(8-4-22-81-72(77)78)60(107)92-48-31-122-121-30-47-64(111)94-50(67(114)95-57)33-124-123-32-49(93-61(108)42(17-19-53(76)100)86-62(109)45(27-36-13-18-52(99)38(74)25-36)89-59(106)40(85-65(48)112)9-5-23-82-73(79)120)66(113)90-44(26-35-11-14-37(98)15-12-35)63(110)88-43(71(118)119)7-2-3-21-80-54(101)20-16-39(75)58(105)83-29-55(102)84-47/h11-15,18,25,34,39-51,57,97-99H,2-10,16-17,19-24,26-33,75H2,1H3,(H2,76,100)(H,80,101)(H,83,105)(H,84,102)(H,85,112)(H,86,109)(H,87,115)(H,88,110)(H,89,106)(H,90,113)(H,91,116)(H,92,107)(H,93,108)(H,94,111)(H,95,114)(H,103,104)(H,118,119)(H4,77,78,81)(H3,79,82,120)/t34-,39+,40+,41+,42+,43-,44+,45+,46+,47+,48+,49+,50+,51+,57+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha9alpha10 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of Ach-induced response measured after 5 ... |
J Med Chem 63: 8380-8387 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00613
BindingDB Entry DOI: 10.7270/Q2474FDJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50581246

(CHEMBL5080428)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H]2NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]3CCCN3C(=O)[C@H](CC(O)=O)NC1=O)C(=O)N[C@@H](CCCNC(N)=O)C(=O)N[C@@H](Cc1ccc(O)c(I)c1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CSCS2)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)CN |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human alpha9alpha10 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced current response by two-e... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00802
BindingDB Entry DOI: 10.7270/Q2542SG3 |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326749

((3S,3aR,4S,6S,6aR,7S,8S,9bS)-6-acetoxy-4-(butyrylo...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)c2cccc(C)c2)C(C)=C2[C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@](C)(OC(C)=O)[C@@H]12)OC(=O)CCC |r,t:24| Show InChI InChI=1S/C37H50O12/c1-8-10-11-12-13-18-27(40)46-31-29-28(22(4)30(31)47-33(41)24-17-14-16-21(3)19-24)32-37(44,36(7,43)34(42)48-32)25(45-26(39)15-9-2)20-35(29,6)49-23(5)38/h14,16-17,19,25,29-32,43-44H,8-13,15,18,20H2,1-7H3/t25-,29+,30-,31-,32-,35-,36+,37+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326742
((3S,3aR,4S,6S,6aR,7S,8S,9bS)-6-acetoxy-3,3a,4-trih...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)C(\C)=C/C)C(C)=C2[C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@@H](O)C[C@](C)(OC(C)=O)[C@@H]12 |r,t:20| Show InChI InChI=1S/C30H44O11/c1-8-10-11-12-13-14-20(33)38-24-22-21(17(4)23(24)39-26(34)16(3)9-2)25-30(37,29(7,36)27(35)40-25)19(32)15-28(22,6)41-18(5)31/h9,19,22-25,32,36-37H,8,10-15H2,1-7H3/b16-9-/t19-,22+,23-,24-,25-,28-,29+,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326746
((3S,3aR,4S,6S,6aR,7S,8S,9bS)-3a-hydroxy-3,6,9-trim...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)C(\C)=C/C)C(C)=C2[C@@H]3OC(=O)[C@@](C)(OC(=O)CCC)[C@@]3(O)[C@H](C[C@](C)(OC(=O)CCC)[C@@H]12)OC(=O)CCC |r,t:20| Show InChI InChI=1S/C40H60O13/c1-10-15-16-17-18-22-28(42)49-34-32-31(25(7)33(34)50-36(45)24(6)14-5)35-40(47,39(9,37(46)51-35)53-30(44)21-13-4)26(48-27(41)19-11-2)23-38(32,8)52-29(43)20-12-3/h14,26,32-35,47H,10-13,15-23H2,1-9H3/b24-14-/t26-,32+,33-,34-,35-,38-,39+,40+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50544006

(CHEMBL4634288)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H]2CSSC[C@@H]3NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc4ccc(O)cc4)NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@H](CSSC[C@H](NC(=O)CNC(=O)[C@@H](N)CCC(=O)NCCCC[C@@H](NC(=O)[C@H](Cc4ccc(O)cc4)NC3=O)C(O)=O)C(=O)N2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(O)=O)NC1=O |r| Show InChI InChI=1S/C73H106N22O24S4/c1-35(96)57-69(115)90-47(29-56(102)103)70(116)95-26-6-10-52(95)68(114)86-42(8-4-24-80-72(76)77)60(106)91-49-32-121-120-31-48-64(110)93-51(67(113)94-57)34-123-122-33-50(92-61(107)43(20-21-53(75)99)85-62(108)45(27-36-11-15-38(97)16-12-36)88-59(105)41(84-65(49)111)9-5-25-81-73(78)119)66(112)89-46(28-37-13-17-39(98)18-14-37)63(109)87-44(71(117)118)7-2-3-23-79-54(100)22-19-40(74)58(104)82-30-55(101)83-48/h11-18,35,40-52,57,96-98H,2-10,19-34,74H2,1H3,(H2,75,99)(H,79,100)(H,82,104)(H,83,101)(H,84,111)(H,85,108)(H,86,114)(H,87,109)(H,88,105)(H,89,112)(H,90,115)(H,91,106)(H,92,107)(H,93,110)(H,94,113)(H,102,103)(H,117,118)(H4,76,77,80)(H3,78,81,119)/t35-,40+,41+,42+,43+,44-,45+,46+,47+,48+,49+,50+,51+,52+,57+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha9alpha10 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of Ach-induced response measured after 5 ... |
J Med Chem 63: 8380-8387 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00613
BindingDB Entry DOI: 10.7270/Q2474FDJ |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326739

((3S,3aR,4S,6S,6aR,7S,8S,9bS)-4-(butyryloxy)-3,3a-d...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)C(\C)=C/C)C(C)=C2[C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@](C)(OC(=O)c3ccccc3)[C@@H]12)OC(=O)CCC |r,t:20| Show InChI InChI=1S/C39H52O12/c1-8-11-12-13-17-21-28(41)48-32-30-29(24(5)31(32)49-34(42)23(4)10-3)33-39(46,38(7,45)36(44)50-33)26(47-27(40)18-9-2)22-37(30,6)51-35(43)25-19-15-14-16-20-25/h10,14-16,19-20,26,30-33,45-46H,8-9,11-13,17-18,21-22H2,1-7H3/b23-10-/t26-,30+,31-,32-,33-,37-,38+,39+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326738
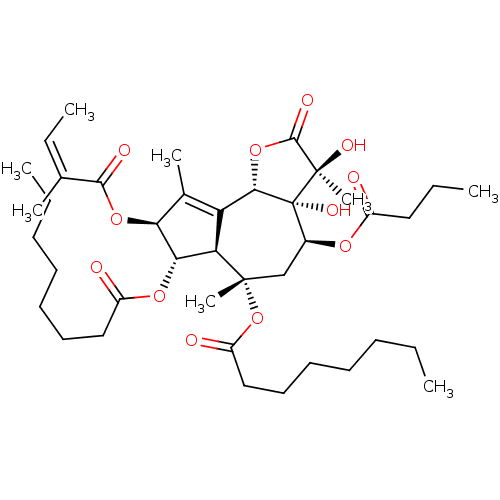
((3S,3aR,4S,6S,6aR,7S,8S,9bS)-4-(butyryloxy)-3,3a-d...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)C(\C)=C/C)C(C)=C2[C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@](C)(OC(=O)CCCCCCC)[C@@H]12)OC(=O)CCC |r,t:20| Show InChI InChI=1S/C40H62O12/c1-9-13-15-17-19-22-29(42)49-34-32-31(26(6)33(34)50-36(44)25(5)12-4)35-40(47,39(8,46)37(45)51-35)27(48-28(41)21-11-3)24-38(32,7)52-30(43)23-20-18-16-14-10-2/h12,27,32-35,46-47H,9-11,13-24H2,1-8H3/b25-12-/t27-,32+,33-,34-,35-,38-,39+,40+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326750

((3S,3aR,4S,6S,6aR,7S,8S,9bS)-6-acetoxy-8-(2-(biphe...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)Cc2ccc(cc2)-c2ccccc2)C(C)=C2[C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@](C)(OC(C)=O)[C@@H]12)OC(=O)CCC |r,t:31| Show InChI InChI=1S/C43H54O12/c1-7-9-10-11-15-19-33(46)52-38-36-35(26(3)37(38)53-34(47)24-28-20-22-30(23-21-28)29-17-13-12-14-18-29)39-43(50,42(6,49)40(48)54-39)31(51-32(45)16-8-2)25-41(36,5)55-27(4)44/h12-14,17-18,20-23,31,36-39,49-50H,7-11,15-16,19,24-25H2,1-6H3/t31-,36+,37-,38-,39-,41-,42+,43+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326745

(CHEMBL1253588 | Octanoic acid(2aS,5aS,7S,7aR,8S,9S...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)C(\C)=C/C)C(C)=C2[C@@H]3OC(=O)[C@@]4(C)OC(C)(C)O[C@@H](C[C@](C)(OC(=O)CCC)[C@@H]12)[C@@]34O |r,t:20| Show InChI InChI=1S/C35H52O11/c1-10-13-14-15-16-18-23(36)41-28-26-25(21(5)27(28)42-30(38)20(4)12-3)29-35(40)22(19-33(26,8)45-24(37)17-11-2)44-32(6,7)46-34(35,9)31(39)43-29/h12,22,26-29,40H,10-11,13-19H2,1-9H3/b20-12-/t22-,26+,27-,28-,29-,33-,34+,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Homo sapiens (Human)) | BDBM50165366

(CHEMBL191927 | Pentanedioic acid mono-((2S,3aR,4S,...)Show SMILES CCCCCCCC(=O)O[C@H]1[C@H]2C([C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@]2(C)OC(C)=O)OC(=O)CCCC(O)=O)=C(C)C1=O |t:40| Show InChI InChI=1S/C30H42O13/c1-6-7-8-9-10-13-21(35)41-25-23-22(16(2)24(25)36)26-30(39,29(5,38)27(37)42-26)18(15-28(23,4)43-17(3)31)40-20(34)14-11-12-19(32)33/h18,23,25-26,38-39H,6-15H2,1-5H3,(H,32,33)/t18-,23+,25-,26-,28-,29+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University
Curated by ChEMBL
| Assay Description
Inhibitory activity against sarco- endoplasmic reticulum calcium-ATPase |
J Med Chem 48: 3005-14 (2005)
Article DOI: 10.1021/jm049319a
BindingDB Entry DOI: 10.7270/Q27W6BP4 |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326736

((3S,3aR,4S,6S,6aR,7S,8S,9bS)-3,3a,6-trihydroxy-3,6...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)C(\C)=C/C)C(C)=C2[C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@](C)(O)[C@@H]12)OC(=O)CCCCCCC |r,t:20| Show InChI InChI=1S/C36H56O11/c1-8-11-13-15-17-19-25(37)44-24-21-34(6,41)28-27(31-36(24,43)35(7,42)33(40)47-31)23(5)29(46-32(39)22(4)10-3)30(28)45-26(38)20-18-16-14-12-9-2/h10,24,28-31,41-43H,8-9,11-21H2,1-7H3/b22-10-/t24-,28+,29-,30-,31-,34-,35+,36+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50530304
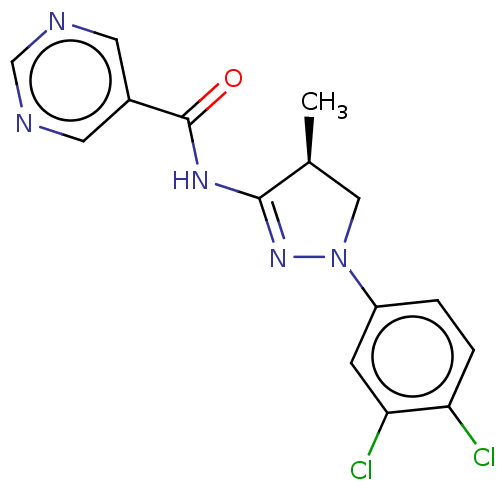
(CHEMBL4445989)Show SMILES C[C@H]1CN(N=C1NC(=O)c1cncnc1)c1ccc(Cl)c(Cl)c1 |r,c:4| Show InChI InChI=1S/C15H13Cl2N5O/c1-9-7-22(11-2-3-12(16)13(17)4-11)21-14(9)20-15(23)10-5-18-8-19-6-10/h2-6,8-9H,7H2,1H3,(H,20,21,23)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Activation of full length human 6His-tagged c-Abl expressed in baculovirus expression system using abltide as substrate after 2 hrs by IMAP assay |
J Med Chem 62: 2154-2171 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01872
BindingDB Entry DOI: 10.7270/Q2X92FR3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50530304

(CHEMBL4445989)Show SMILES C[C@H]1CN(N=C1NC(=O)c1cncnc1)c1ccc(Cl)c(Cl)c1 |r,c:4| Show InChI InChI=1S/C15H13Cl2N5O/c1-9-7-22(11-2-3-12(16)13(17)4-11)21-14(9)20-15(23)10-5-18-8-19-6-10/h2-6,8-9H,7H2,1H3,(H,20,21,23)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Activation of full length human 6His-tagged c-Abl expressed in baculovirus expression system using abltide as substrate after 2 hrs by IMAP assay |
J Med Chem 62: 2154-2171 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01872
BindingDB Entry DOI: 10.7270/Q2X92FR3 |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326735
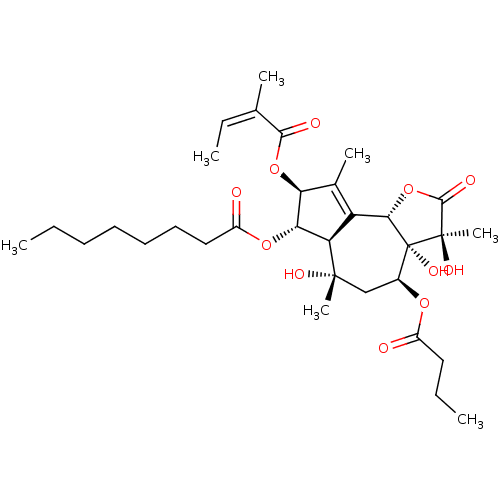
((3S,3aR,4S,6S,6aR,7S,8S,9bS)-4-(butyryloxy)-3,3a,6...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)C(\C)=C/C)C(C)=C2[C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@](C)(O)[C@@H]12)OC(=O)CCC |r,t:20| Show InChI InChI=1S/C32H48O11/c1-8-11-12-13-14-16-22(34)41-26-24-23(19(5)25(26)42-28(35)18(4)10-3)27-32(39,31(7,38)29(36)43-27)20(17-30(24,6)37)40-21(33)15-9-2/h10,20,24-27,37-39H,8-9,11-17H2,1-7H3/b18-10-/t20-,24+,25-,26-,27-,30-,31+,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606648
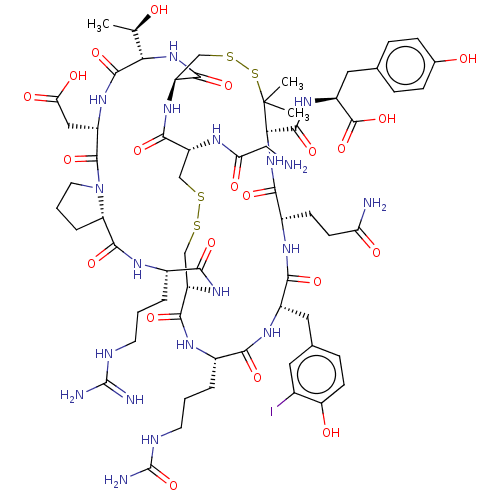
(CHEMBL5220622)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H]1CSSC(C)(C)[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc3ccc(O)c(I)c3)NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@H](CSSC[C@@H](NC(=O)CN)C(=O)N1)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1
(Oryctolagus cuniculus) | BDBM50326741
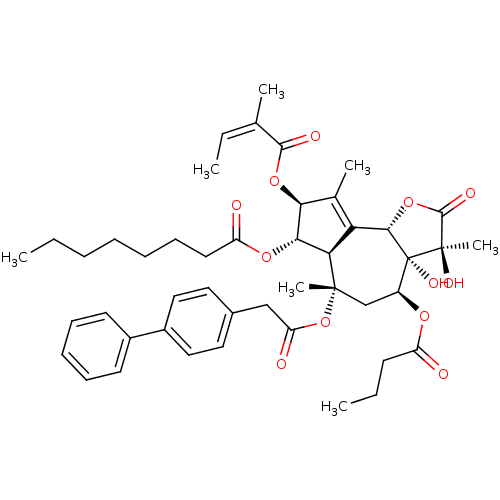
((3S,3aR,4S,6S,6aR,7S,8S,9bS)-6-(2-(biphenyl-4-yl)a...)Show SMILES CCCCCCCC(=O)O[C@@H]1[C@@H](OC(=O)C(\C)=C/C)C(C)=C2[C@@H]3OC(=O)[C@@](C)(O)[C@@]3(O)[C@H](C[C@](C)(OC(=O)Cc3ccc(cc3)-c3ccccc3)[C@@H]12)OC(=O)CCC |r,t:20| Show InChI InChI=1S/C46H58O12/c1-8-11-12-13-17-21-35(48)55-40-38-37(29(5)39(40)56-42(50)28(4)10-3)41-46(53,45(7,52)43(51)57-41)33(54-34(47)18-9-2)27-44(38,6)58-36(49)26-30-22-24-32(25-23-30)31-19-15-14-16-20-31/h10,14-16,19-20,22-25,33,38-41,52-53H,8-9,11-13,17-18,21,26-27H2,1-7H3/b28-10-/t33-,38+,39-,40-,41-,44-,45+,46+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sarcoplasmic reticulum calcium ATPase by ATP regeneration assay |
Bioorg Med Chem 18: 5634-46 (2010)
Article DOI: 10.1016/j.bmc.2010.06.032
BindingDB Entry DOI: 10.7270/Q2RB74VD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50530295
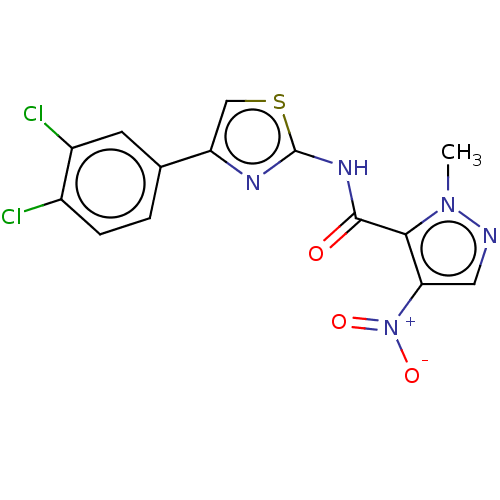
(CHEMBL4453927)Show SMILES Cn1ncc(c1C(=O)Nc1nc(cs1)-c1ccc(Cl)c(Cl)c1)[N+]([O-])=O Show InChI InChI=1S/C14H9Cl2N5O3S/c1-20-12(11(5-17-20)21(23)24)13(22)19-14-18-10(6-25-14)7-2-3-8(15)9(16)4-7/h2-6H,1H3,(H,18,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Activation of full length human 6His-tagged c-Abl expressed in baculovirus expression system using abltide as substrate after 2 hrs by IMAP assay |
J Med Chem 62: 2154-2171 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01872
BindingDB Entry DOI: 10.7270/Q2X92FR3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50530295

(CHEMBL4453927)Show SMILES Cn1ncc(c1C(=O)Nc1nc(cs1)-c1ccc(Cl)c(Cl)c1)[N+]([O-])=O Show InChI InChI=1S/C14H9Cl2N5O3S/c1-20-12(11(5-17-20)21(23)24)13(22)19-14-18-10(6-25-14)7-2-3-8(15)9(16)4-7/h2-6H,1H3,(H,18,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Activation of full length human 6His-tagged c-Abl expressed in baculovirus expression system using abltide as substrate after 2 hrs by IMAP assay |
J Med Chem 62: 2154-2171 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01872
BindingDB Entry DOI: 10.7270/Q2X92FR3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor; alpha9/alpha10
(Homo sapiens (Human)) | BDBM50606646
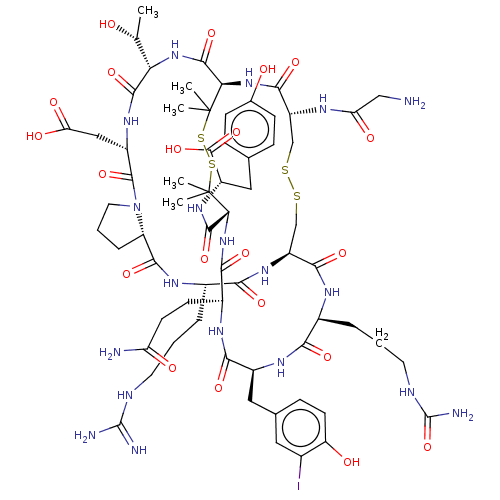
(CHEMBL5221023)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=O)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](Cc2ccc(O)cc2)C(O)=O)C(C)(C)SSC1(C)C)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data