

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM50423452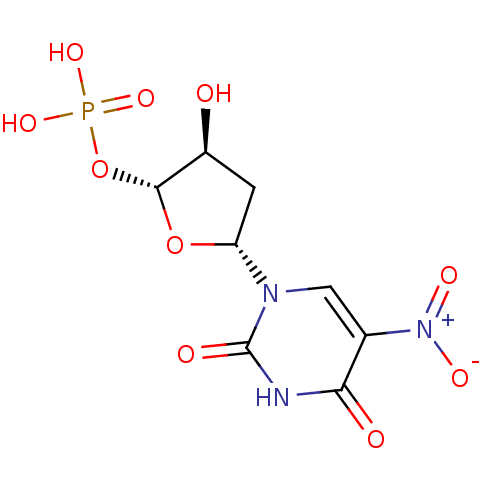 (CHEMBL401166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50084626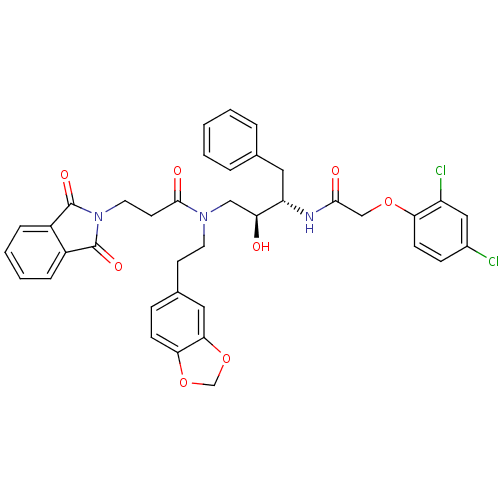 (CHEMBL284440 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50110934 (CHEMBL30483 | N-((1S,3S)-1-Benzyl-3-{[2-(2,4-dichl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50423448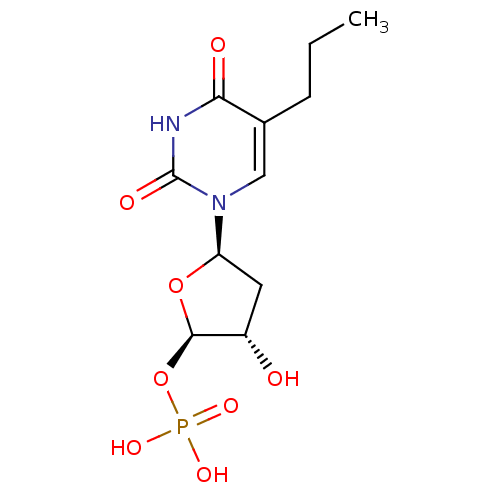 (CHEMBL254204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50423449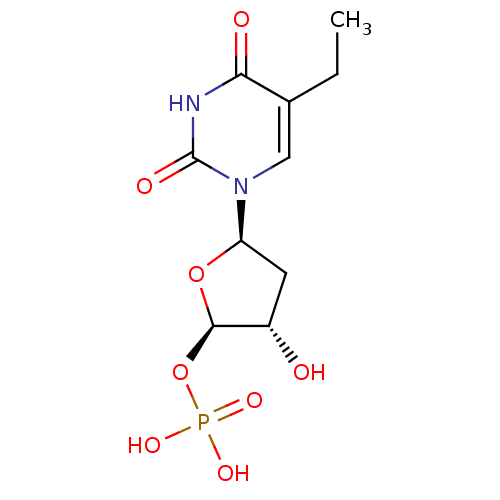 (CHEMBL253999) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50110932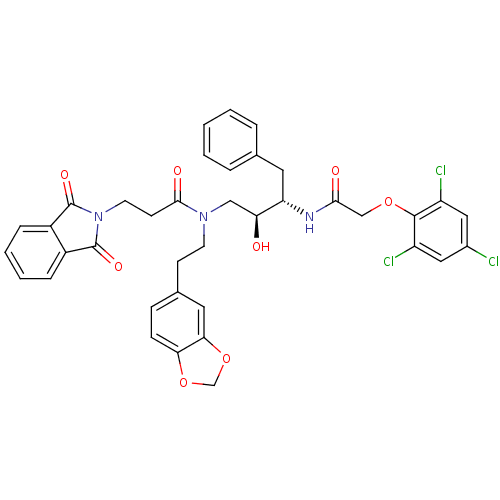 (CHEMBL283863 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50423450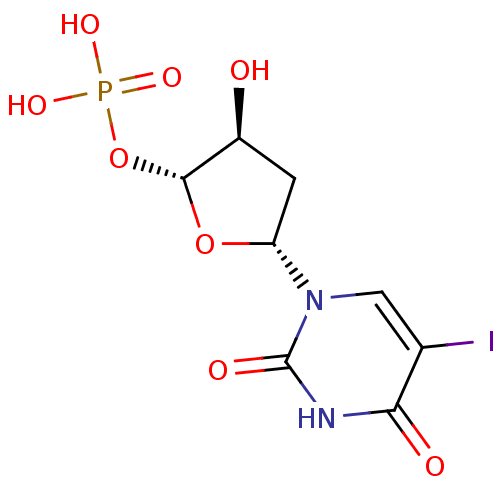 (CHEMBL251934) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50423455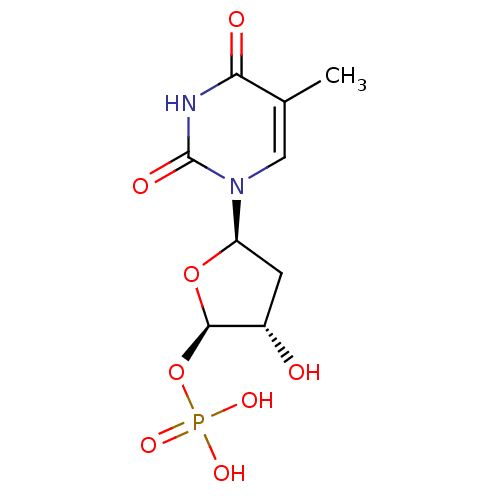 (CHEMBL399050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50110931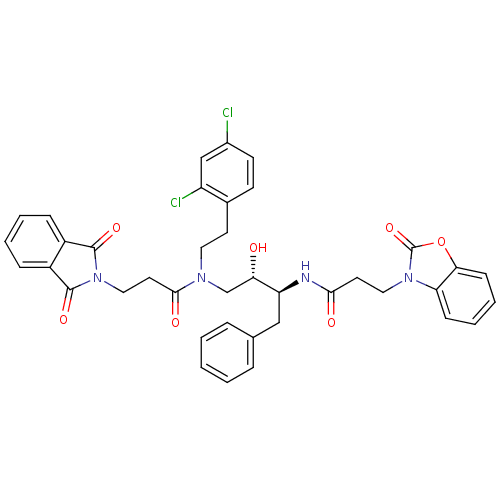 (CHEMBL284441 | N-((1S,3S)-1-Benzyl-3-{[2-(2,4-dich...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50423454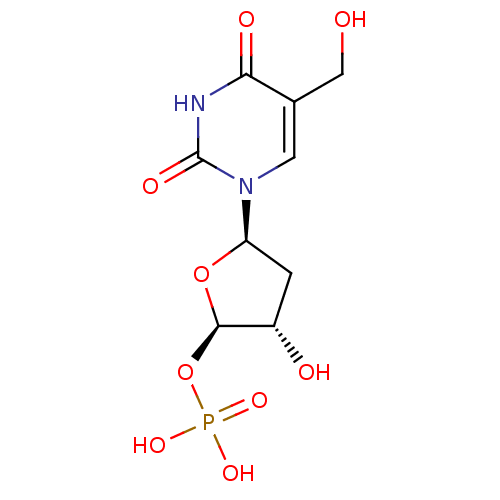 (CHEMBL400683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50423457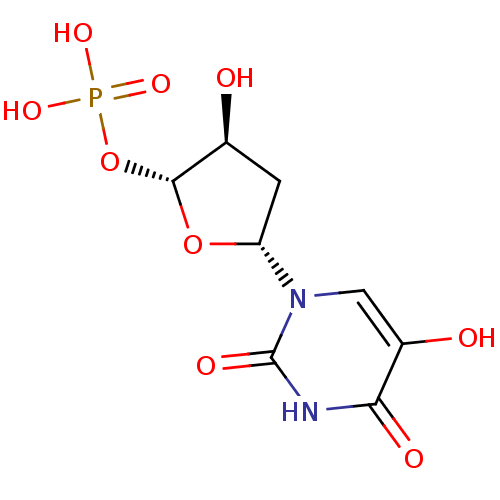 (CHEMBL251255) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50423453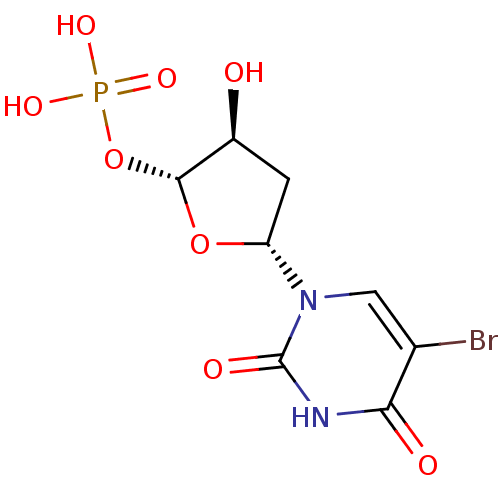 (CHEMBL401165) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50110933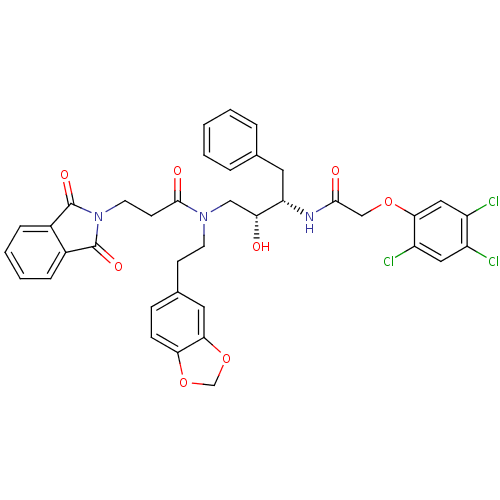 (CHEMBL30571 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50110935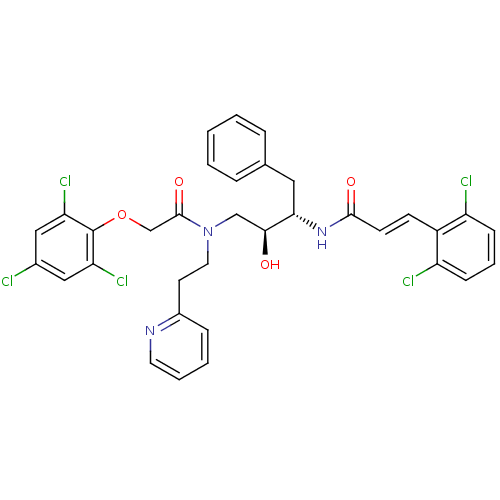 ((E)-N-((1S,3S)-1-Benzyl-2-hydroxy-3-{(2-pyridin-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50423451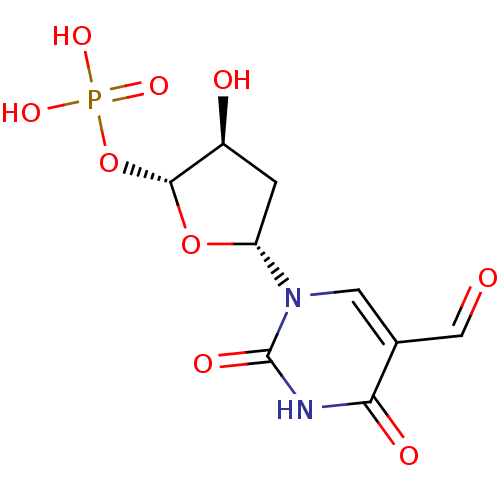 (CHEMBL254205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50423447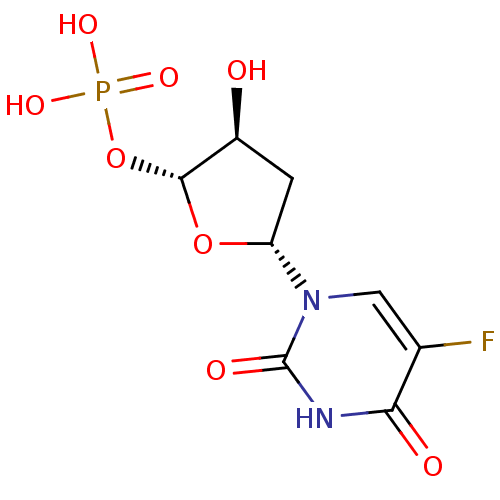 (CHEMBL255049) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50423456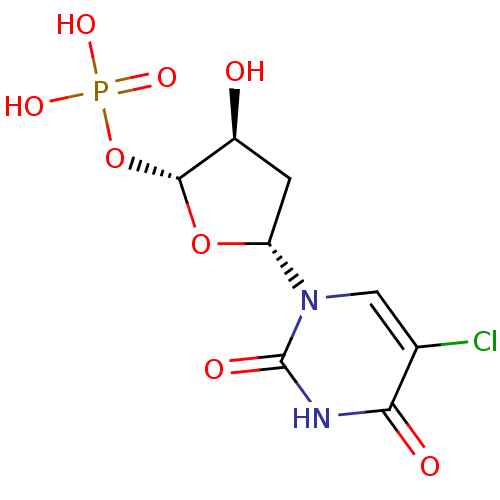 (CHEMBL255050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.02E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24572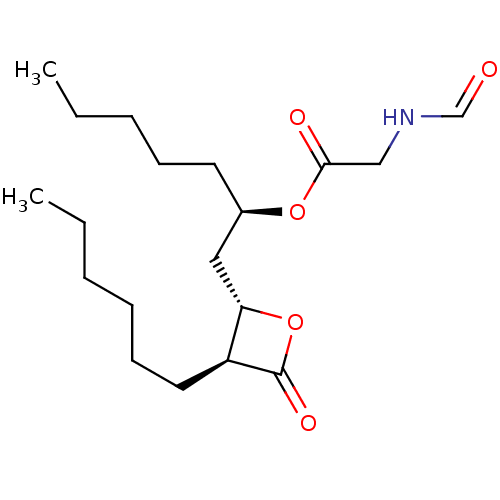 ((2R)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]heptan-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24573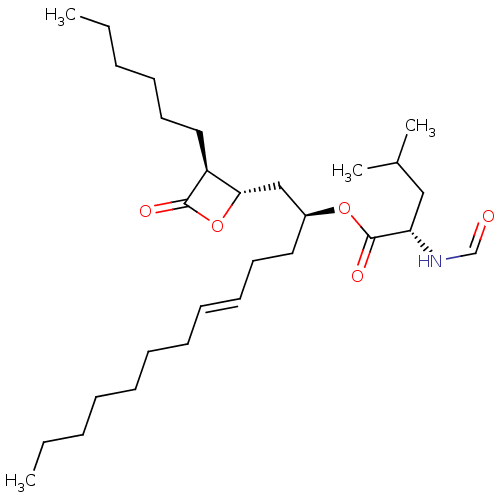 ((2S,5E)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridec...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24574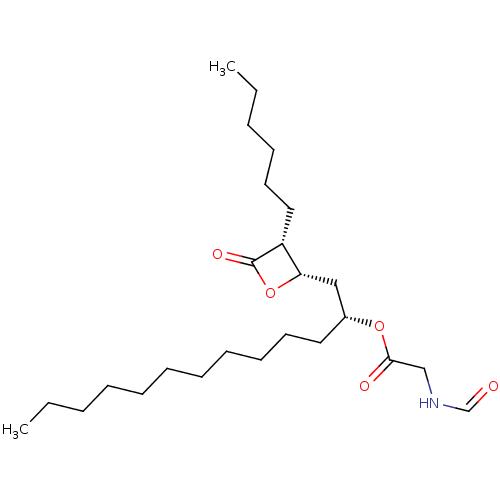 ((2R)-1-[(2S,3R)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24575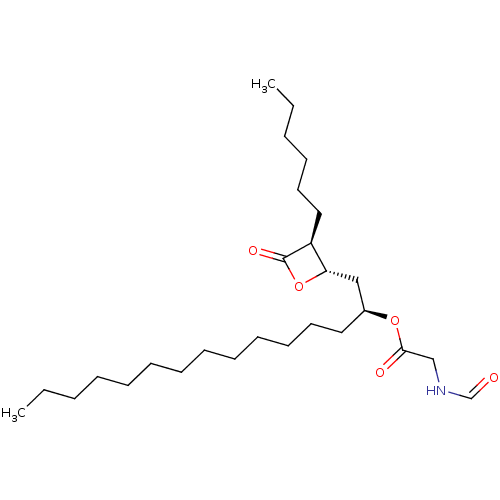 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]pentadeca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24569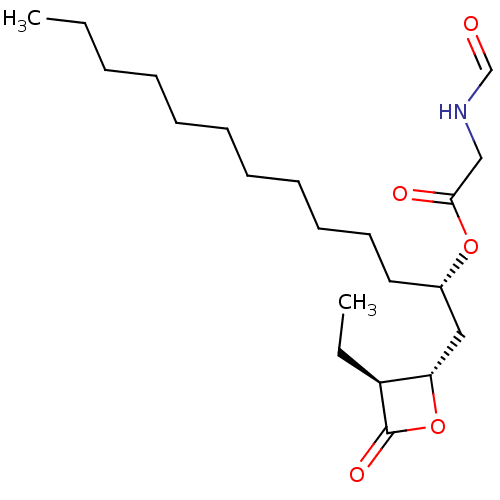 ((1R)-1-{[(2S,3S)-3-ethyl-4-oxooxetan-2-yl]methyl}d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24570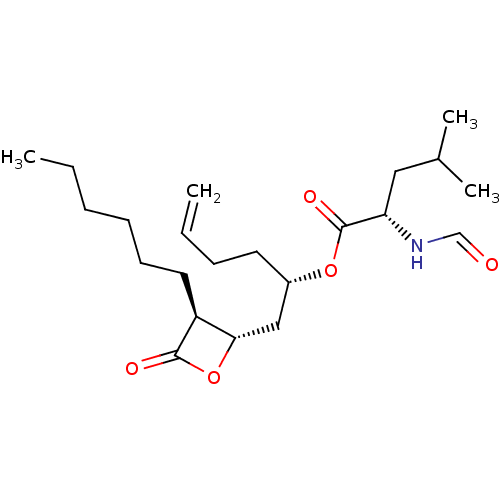 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]hex-5-en-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24576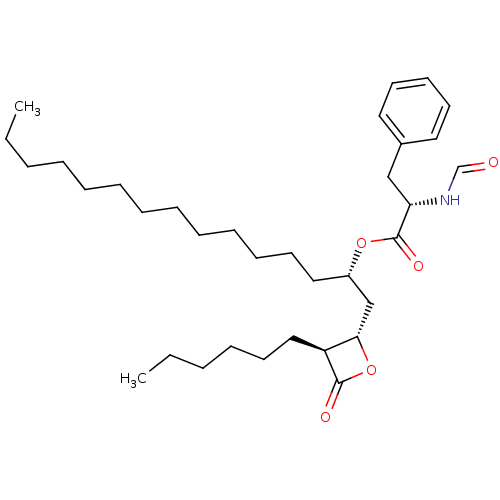 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]pentadeca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24571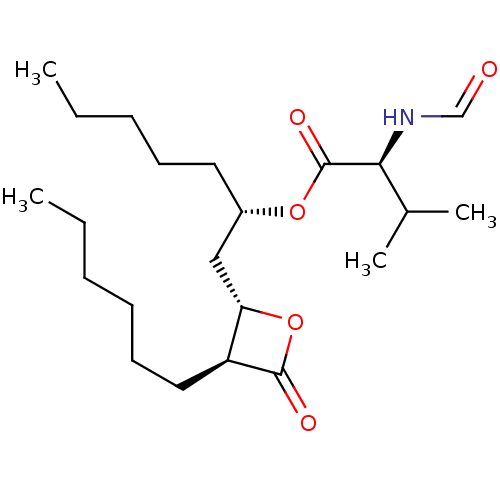 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]heptan-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24577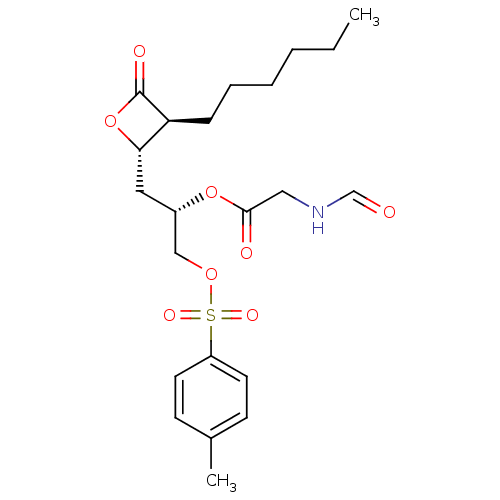 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]-3-{[(4-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24578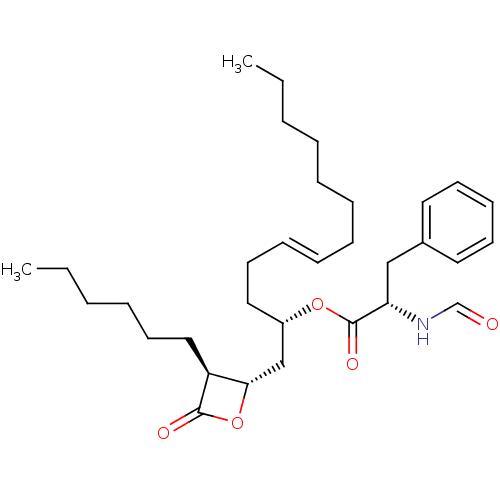 ((2S,5E)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridec...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24579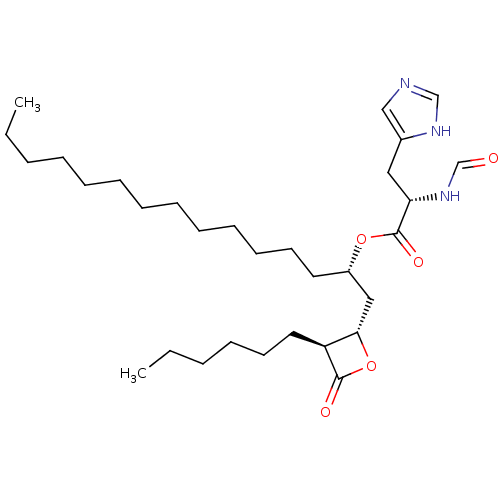 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]pentadeca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24580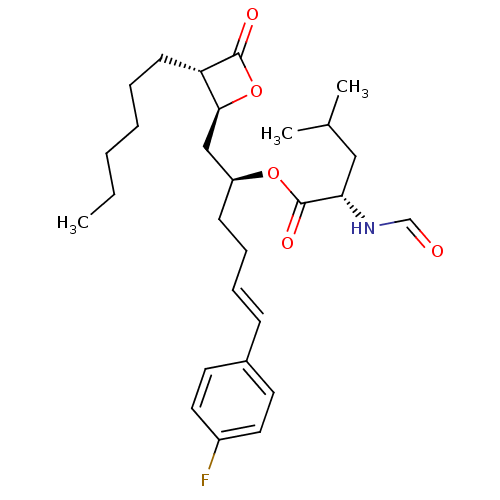 ((2S,5E)-6-(4-fluorophenyl)-1-[(2S,3S)-3-hexyl-4-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24581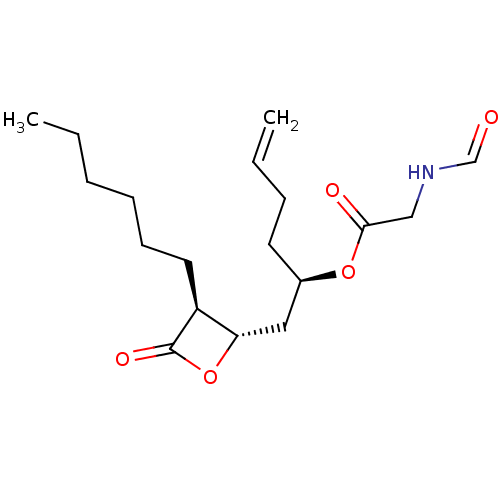 ((2R)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]hex-5-en-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24582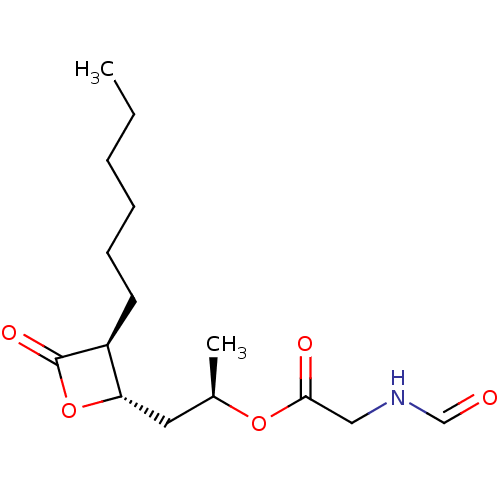 ((2R)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]propan-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24583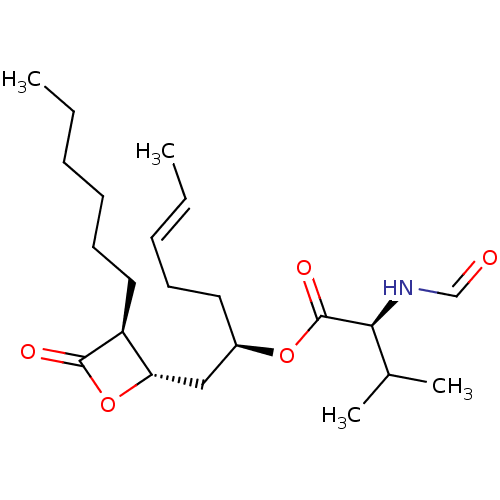 ((2R,5E)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]hept-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24569 ((1R)-1-{[(2S,3S)-3-ethyl-4-oxooxetan-2-yl]methyl}d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24585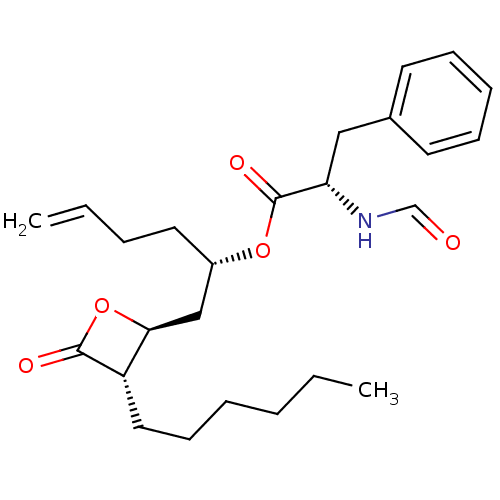 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]hex-5-en-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24586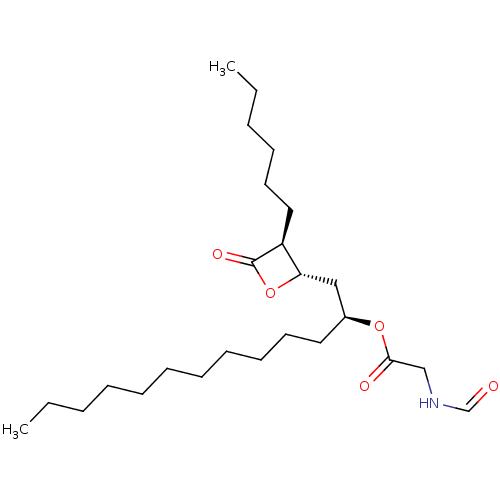 ((1R)-1-{[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]methyl}d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24587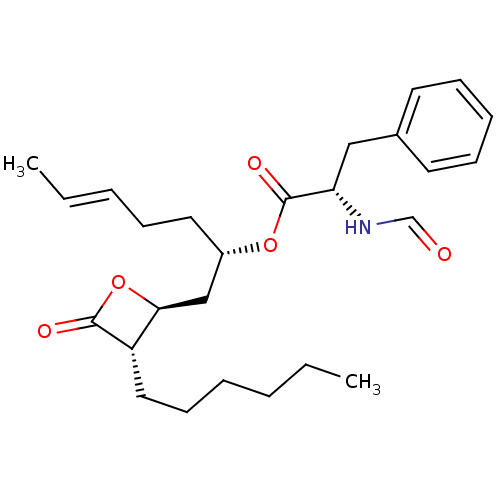 ((2S,5E)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]hept-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24567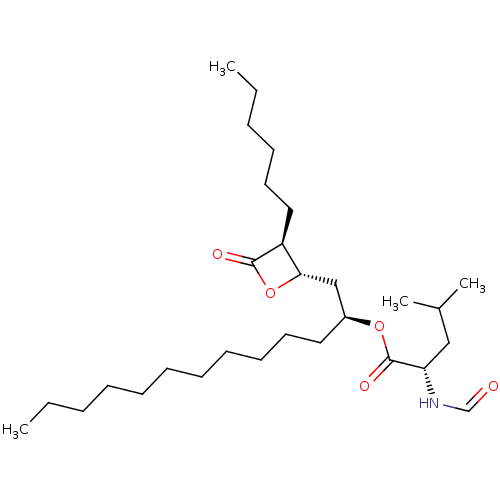 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24588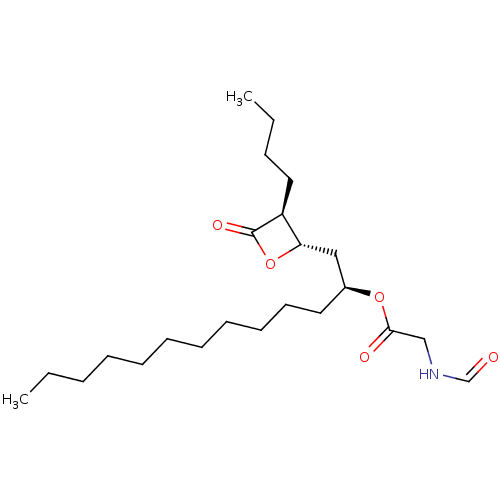 ((1R)-1-{[(2S,3S)-3-butyl-4-oxooxetan-2-yl]methyl}d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24586 ((1R)-1-{[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]methyl}d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24590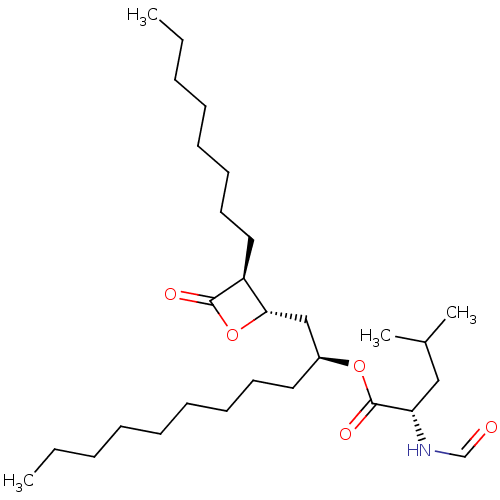 ((2S)-1-[(2S,3S)-3-octyl-4-oxooxetan-2-yl]undecan-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24591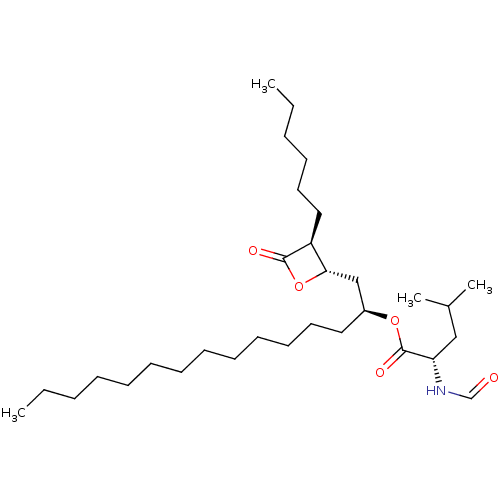 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]pentadeca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.67E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24592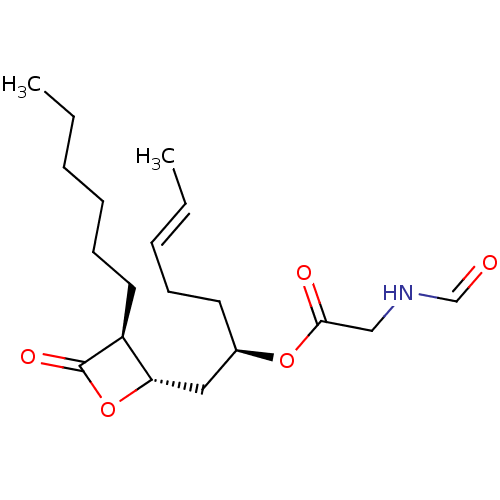 ((2R,5E)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]hept-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.24E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24593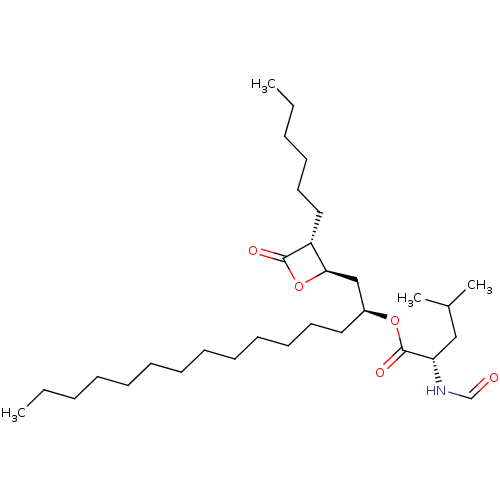 ((2S)-1-[(2R,3R)-3-hexyl-4-oxooxetan-2-yl]pentadeca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.74E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24594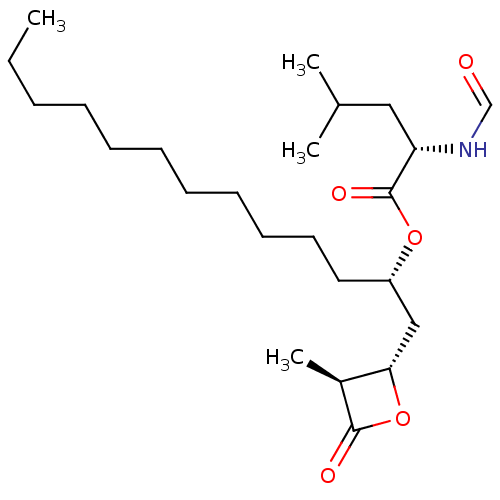 ((2S)-1-[(2S,3S)-3-methyl-4-oxooxetan-2-yl]tridecan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24595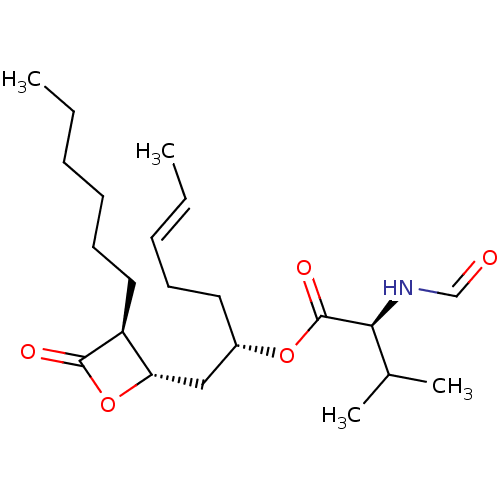 ((2S,5E)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]hept-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase [2202-2509] (Homo sapiens (Human)) | BDBM24596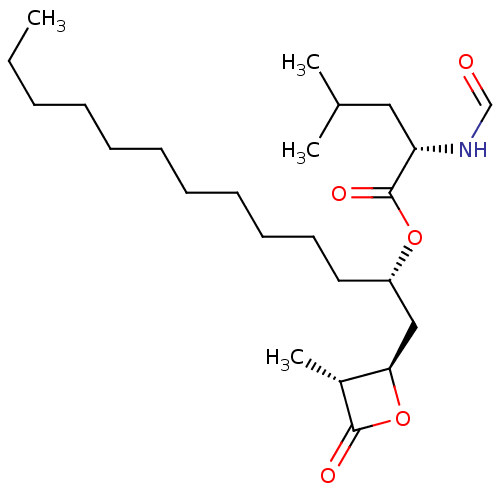 ((2S)-1-[(2R,3R)-3-methyl-4-oxooxetan-2-yl]tridecan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.51E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
The Burnham Institute for Medical Research | Assay Description The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ... | J Med Chem 51: 5285-96 (2008) Article DOI: 10.1021/jm800321h BindingDB Entry DOI: 10.7270/Q2NV9GJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||