

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of recombinant human AChE assessed as inhibition constant using acetylthiocholine iodide as substrate by Cornish-Bowden plot an... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of recombinant human AChE assessed as dissociation constant for protein-substrate-compound complex using acetylthiocholine iodi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556958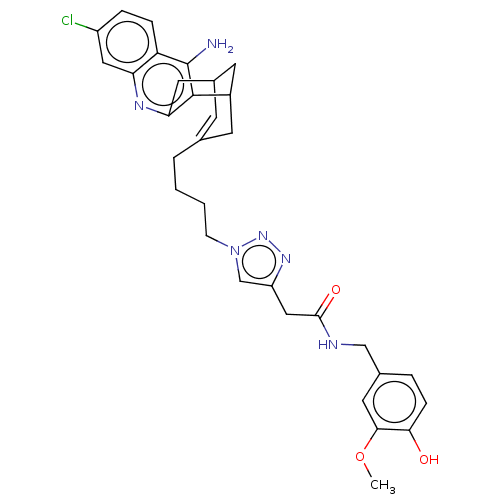 (CHEMBL4744528) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556953 (CHEMBL4748114) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556954 (CHEMBL4782921) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556956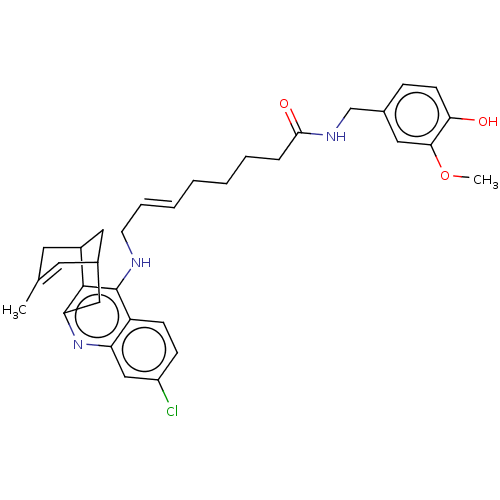 (CHEMBL4741162) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556951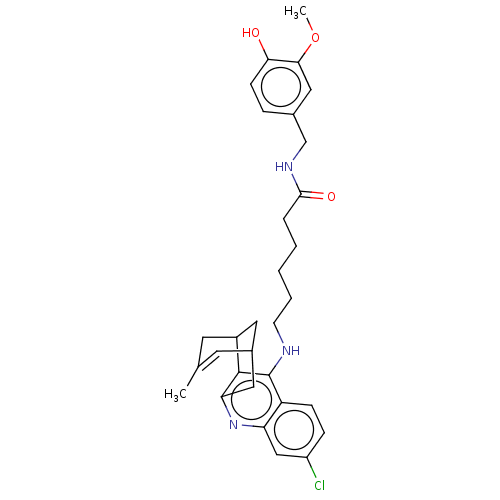 (CHEMBL4788434) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556950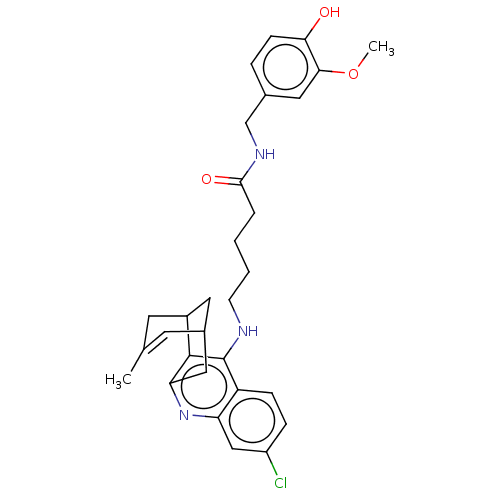 (CHEMBL4743210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556957 (CHEMBL4794769) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556958 (CHEMBL4744528) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556955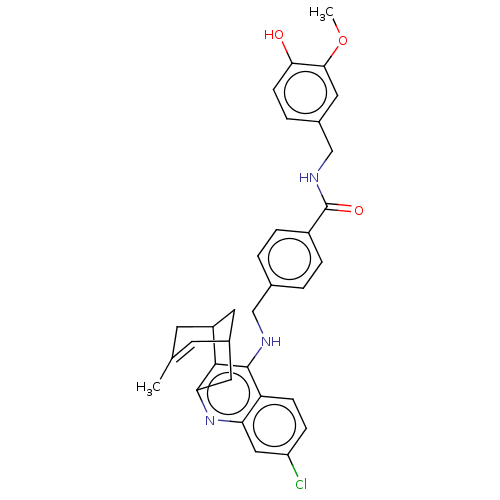 (CHEMBL4782267) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556954 (CHEMBL4782921) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556953 (CHEMBL4748114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556950 (CHEMBL4743210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556957 (CHEMBL4794769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556951 (CHEMBL4788434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556956 (CHEMBL4741162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50556955 (CHEMBL4782267) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM20461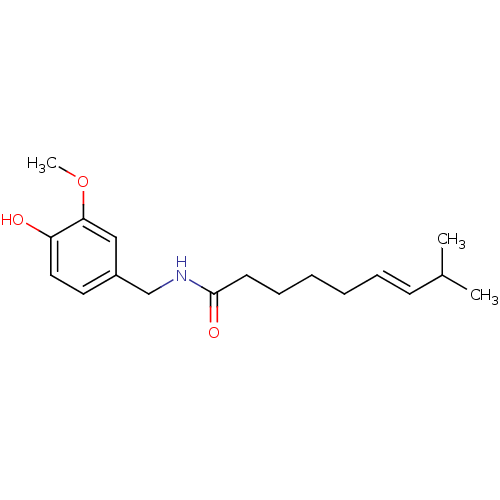 ((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of recombinant human AChE using varying concentration of acetylthiocholine iodide as substrate at 0.17 to 1.36 nM by Lineweaver... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50556952 (CHEMBL4754487) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of propidium iodide from PAS-region of electric eel AChE assessed as dissociation constant by spectrofluorimetric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||