

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM50409115 (LONAPRISAN) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50409115 (LONAPRISAN) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.00360 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-A progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627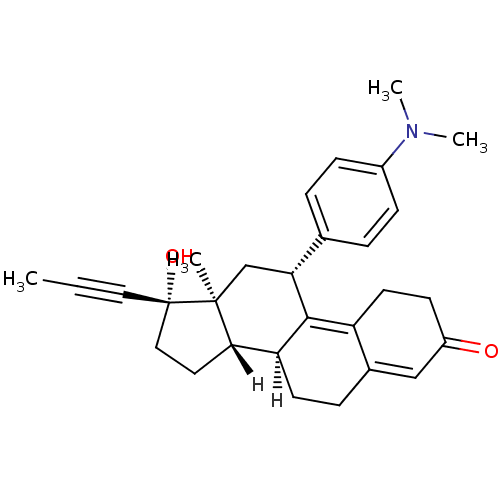 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antgonist potency in transactivation assay in neuroblastoma cells expressing human PR-A progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (MOUSE) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in NIH3T3 cells expressing glucocorticoid receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in CV-1 cells expressing androgen receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (MOUSE) | BDBM50409115 (LONAPRISAN) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in NIH3T3 cells expressing glucocorticoid receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50409115 (LONAPRISAN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in CV-1 cells expressing androgen receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM22416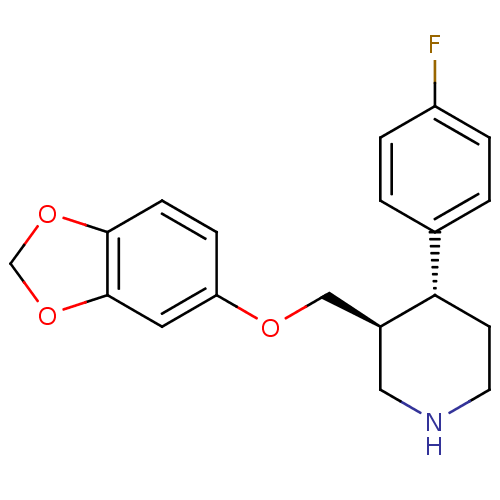 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor | J Med Chem 52: 6107-25 (2009) Article DOI: 10.1021/jm901096y BindingDB Entry DOI: 10.7270/Q2J67HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50034355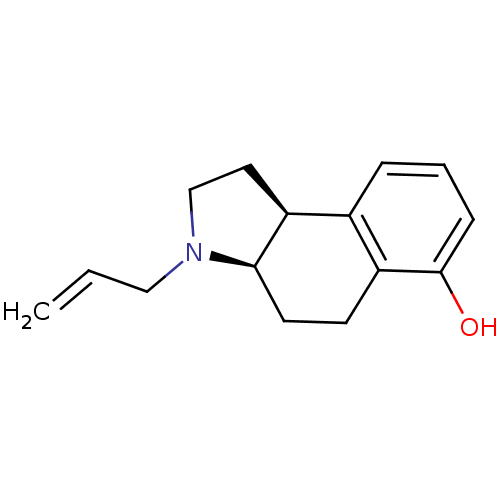 ((3aR,9bS)-3-Allyl-2,3,3a,4,5,9b-hexahydro-1H-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity to 5HT1A receptor | J Med Chem 52: 6107-25 (2009) Article DOI: 10.1021/jm901096y BindingDB Entry DOI: 10.7270/Q2J67HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||