 Found 128 hits with Last Name = 'coleman' and Initial = 's'
Found 128 hits with Last Name = 'coleman' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
UDP-3-O-acyl-N-acetylglucosamine deacetylase
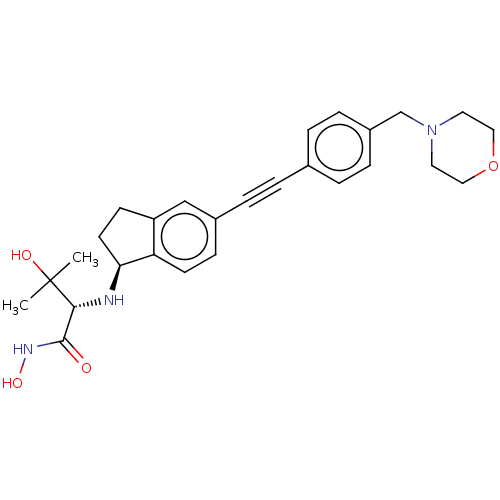
(Escherichia coli) | BDBM50501754

(CHEMBL4071396)Show SMILES C[C@@H](O)[C@H](NC1CCCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H33N3O4/c1-19(31)26(27(32)29-33)28-25-4-2-3-23-17-21(11-12-24(23)25)8-5-20-6-9-22(10-7-20)18-30-13-15-34-16-14-30/h6-7,9-12,17,19,25-26,28,31,33H,2-4,13-16,18H2,1H3,(H,29,32)/t19-,25?,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
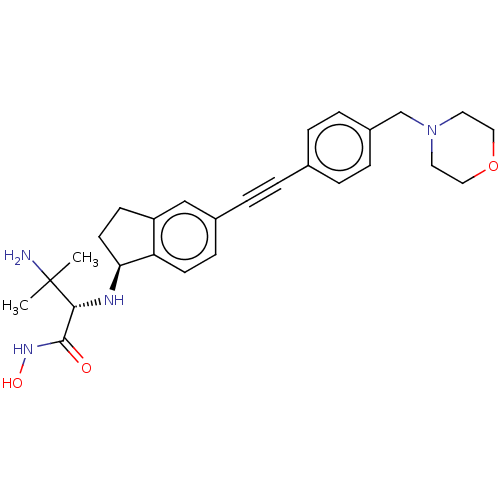
(Pseudomonas aeruginosa) | BDBM50501764
(CHEMBL4061199)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)O |r| Show InChI InChI=1S/C27H33N3O4/c1-27(2,32)25(26(31)29-33)28-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-30-13-15-34-16-14-30/h4-5,7-9,11,17,24-25,28,32-33H,10,12-16,18H2,1-2H3,(H,29,31)/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 847 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
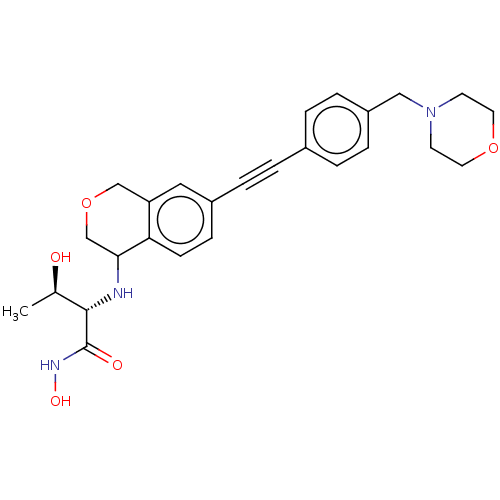
(Pseudomonas aeruginosa) | BDBM50501780
(CHEMBL4083988)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H34N4O3/c1-27(2,28)25(26(32)30-33)29-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-31-13-15-34-16-14-31/h4-5,7-9,11,17,24-25,29,33H,10,12-16,18,28H2,1-2H3,(H,30,32)/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 847 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
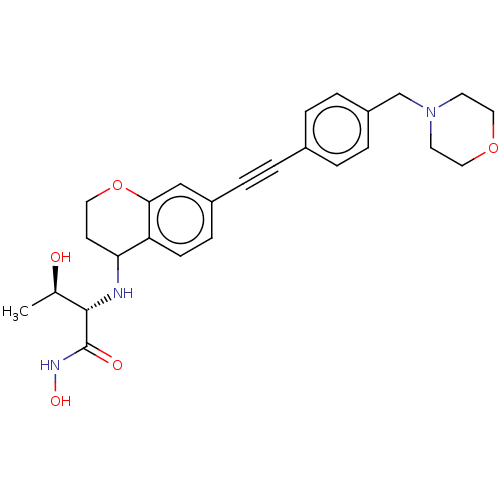
(Escherichia coli) | BDBM50501779
(CHEMBL4102207)Show SMILES C[C@@H](O)[C@H](NC1COCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O5/c1-18(30)25(26(31)28-32)27-24-17-34-16-22-14-20(8-9-23(22)24)5-2-19-3-6-21(7-4-19)15-29-10-12-33-13-11-29/h3-4,6-9,14,18,24-25,27,30,32H,10-13,15-17H2,1H3,(H,28,31)/t18-,24?,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501764

(CHEMBL4061199)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)O |r| Show InChI InChI=1S/C27H33N3O4/c1-27(2,32)25(26(31)29-33)28-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-30-13-15-34-16-14-30/h4-5,7-9,11,17,24-25,28,32-33H,10,12-16,18H2,1-2H3,(H,29,31)/t24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501782

(CHEMBL4087371)Show SMILES C[C@@H](O)[C@H](NC1CCCc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H24N2O3/c1-15(25)21(22(26)24-27)23-20-9-5-8-18-14-17(12-13-19(18)20)11-10-16-6-3-2-4-7-16/h2-4,6-7,12-15,20-21,23,25,27H,5,8-9H2,1H3,(H,24,26)/t15-,20?,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501762

(CHEMBL4081478)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2C)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H28N4O3/c1-17(31)25(26(32)29-33)28-24-12-10-22-15-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)16-30-14-13-27-18(30)2/h4-5,7-9,11,13-15,17,24-25,28,31,33H,10,12,16H2,1-2H3,(H,29,32)/t17-,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501755
(CHEMBL4077947)Show SMILES C[C@@H](O)[C@H](NC1CCOc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O5/c1-18(30)25(26(31)28-32)27-23-10-13-34-24-16-20(8-9-22(23)24)5-2-19-3-6-21(7-4-19)17-29-11-14-33-15-12-29/h3-4,6-9,16,18,23,25,27,30,32H,10-15,17H2,1H3,(H,28,31)/t18-,23?,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501780

(CHEMBL4083988)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H34N4O3/c1-27(2,28)25(26(32)30-33)29-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-31-13-15-34-16-14-31/h4-5,7-9,11,17,24-25,29,33H,10,12-16,18,28H2,1-2H3,(H,30,32)/t24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501778

(CHEMBL4098674)Show SMILES C[C@@H](O)[C@H](NC1COCc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O4/c1-14(24)20(21(25)23-26)22-19-13-27-12-17-11-16(9-10-18(17)19)8-7-15-5-3-2-4-6-15/h2-6,9-11,14,19-20,22,24,26H,12-13H2,1H3,(H,23,25)/t14-,19?,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501770
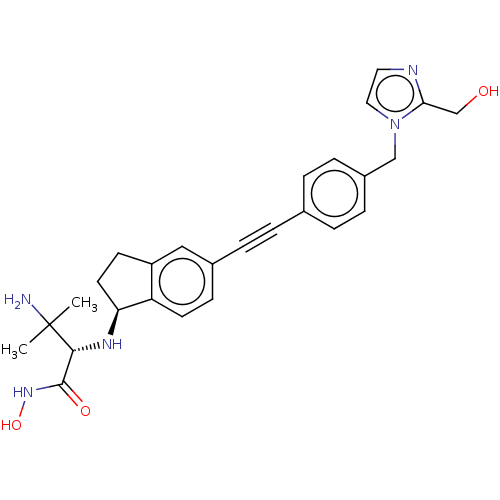
(CHEMBL4092719)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CO)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H31N5O3/c1-27(2,28)25(26(34)31-35)30-23-12-10-21-15-19(9-11-22(21)23)6-3-18-4-7-20(8-5-18)16-32-14-13-29-24(32)17-33/h4-5,7-9,11,13-15,23,25,30,33,35H,10,12,16-17,28H2,1-2H3,(H,31,34)/t23-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 847 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501753

(CHEMBL4098438)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 847 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501752
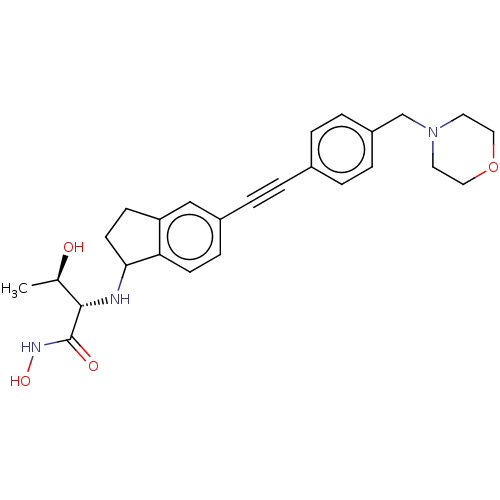
(CHEMBL4095059)Show SMILES C[C@@H](O)[C@H](NC1CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24?,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501754

(CHEMBL4071396)Show SMILES C[C@@H](O)[C@H](NC1CCCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H33N3O4/c1-19(31)26(27(32)29-33)28-25-4-2-3-23-17-21(11-12-24(23)25)8-5-20-6-9-22(10-7-20)18-30-13-15-34-16-14-30/h6-7,9-12,17,19,25-26,28,31,33H,2-4,13-16,18H2,1H3,(H,29,32)/t19-,25?,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501756
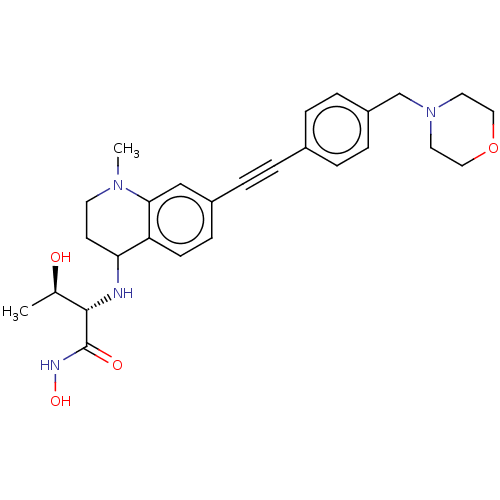
(CHEMBL4066982)Show SMILES C[C@@H](O)[C@H](NC1CCN(C)c2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H34N4O4/c1-19(32)26(27(33)29-34)28-24-11-12-30(2)25-17-21(9-10-23(24)25)6-3-20-4-7-22(8-5-20)18-31-13-15-35-16-14-31/h4-5,7-10,17,19,24,26,28,32,34H,11-16,18H2,1-2H3,(H,29,33)/t19-,24?,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501753

(CHEMBL4098438)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501768
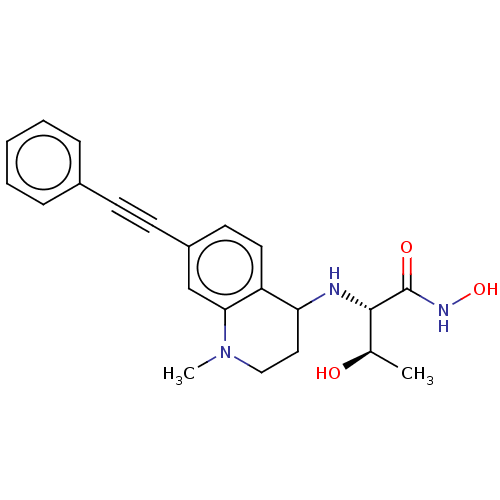
(CHEMBL4102818)Show SMILES C[C@@H](O)[C@H](NC1CCN(C)c2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H25N3O3/c1-15(26)21(22(27)24-28)23-19-12-13-25(2)20-14-17(10-11-18(19)20)9-8-16-6-4-3-5-7-16/h3-7,10-11,14-15,19,21,23,26,28H,12-13H2,1-2H3,(H,24,27)/t15-,19?,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501767

(CHEMBL4091763)Show SMILES CC(C)(N)[C@H](NC1COCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CO)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H31N5O4/c1-27(2,28)25(26(34)31-35)30-23-17-36-16-21-13-19(9-10-22(21)23)6-3-18-4-7-20(8-5-18)14-32-12-11-29-24(32)15-33/h4-5,7-13,23,25,30,33,35H,14-17,28H2,1-2H3,(H,31,34)/t23?,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501764

(CHEMBL4061199)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)O |r| Show InChI InChI=1S/C27H33N3O4/c1-27(2,32)25(26(31)29-33)28-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-30-13-15-34-16-14-30/h4-5,7-9,11,17,24-25,28,32-33H,10,12-16,18H2,1-2H3,(H,29,31)/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501761

(CHEMBL4068010)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCC(CC2)OC)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C28H35N3O4/c1-19(32)27(28(33)30-34)29-26-12-10-23-17-21(9-11-25(23)26)6-3-20-4-7-22(8-5-20)18-31-15-13-24(35-2)14-16-31/h4-5,7-9,11,17,19,24,26-27,29,32,34H,10,12-16,18H2,1-2H3,(H,30,33)/t19-,26+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501753

(CHEMBL4098438)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501783
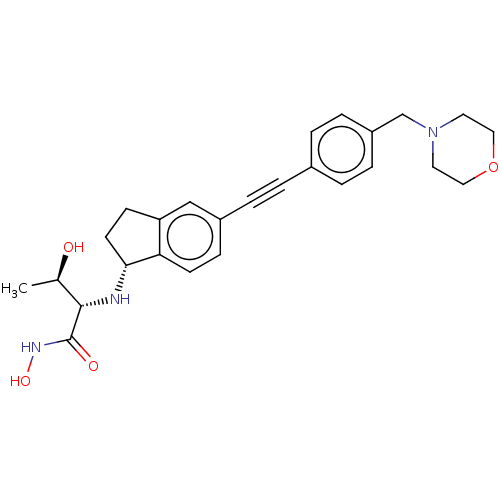
(CHEMBL4064896)Show SMILES [H][C@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24-,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501752

(CHEMBL4095059)Show SMILES C[C@@H](O)[C@H](NC1CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24?,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501770

(CHEMBL4092719)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CO)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H31N5O3/c1-27(2,28)25(26(34)31-35)30-23-12-10-21-15-19(9-11-22(21)23)6-3-18-4-7-20(8-5-18)16-32-14-13-29-24(32)17-33/h4-5,7-9,11,13-15,23,25,30,33,35H,10,12,16-17,28H2,1-2H3,(H,31,34)/t23-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501762

(CHEMBL4081478)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2C)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H28N4O3/c1-17(31)25(26(32)29-33)28-24-12-10-22-15-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)16-30-14-13-27-18(30)2/h4-5,7-9,11,13-15,17,24-25,28,31,33H,10,12,16H2,1-2H3,(H,29,32)/t17-,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501770

(CHEMBL4092719)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CO)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H31N5O3/c1-27(2,28)25(26(34)31-35)30-23-12-10-21-15-19(9-11-22(21)23)6-3-18-4-7-20(8-5-18)16-32-14-13-29-24(32)17-33/h4-5,7-9,11,13-15,23,25,30,33,35H,10,12,16-17,28H2,1-2H3,(H,31,34)/t23-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501782

(CHEMBL4087371)Show SMILES C[C@@H](O)[C@H](NC1CCCc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H24N2O3/c1-15(25)21(22(26)24-27)23-20-9-5-8-18-14-17(12-13-19(18)20)11-10-16-6-3-2-4-7-16/h2-4,6-7,12-15,20-21,23,25,27H,5,8-9H2,1H3,(H,24,26)/t15-,20?,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501766

(CHEMBL4070896)Show SMILES CC(C)(N)[C@H](NC1COCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H34N4O4/c1-27(2,28)25(26(32)30-33)29-24-18-35-17-22-15-20(9-10-23(22)24)6-3-19-4-7-21(8-5-19)16-31-11-13-34-14-12-31/h4-5,7-10,15,24-25,29,33H,11-14,16-18,28H2,1-2H3,(H,30,32)/t24?,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501761

(CHEMBL4068010)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCC(CC2)OC)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C28H35N3O4/c1-19(32)27(28(33)30-34)29-26-12-10-23-17-21(9-11-25(23)26)6-3-20-4-7-22(8-5-20)18-31-15-13-24(35-2)14-16-31/h4-5,7-9,11,17,19,24,26-27,29,32,34H,10,12-16,18H2,1-2H3,(H,30,33)/t19-,26+,27+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501763
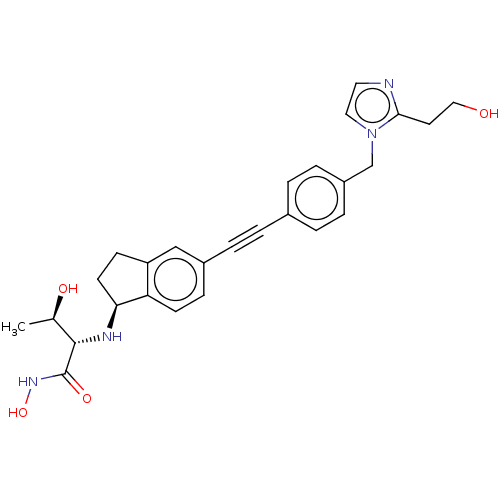
(CHEMBL4099505)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CCO)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C27H30N4O4/c1-18(33)26(27(34)30-35)29-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-31-14-13-28-25(31)12-15-32/h3-4,6-8,10,13-14,16,18,24,26,29,32-33,35H,9,11-12,15,17H2,1H3,(H,30,34)/t18-,24+,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501767

(CHEMBL4091763)Show SMILES CC(C)(N)[C@H](NC1COCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CO)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H31N5O4/c1-27(2,28)25(26(34)31-35)30-23-17-36-16-21-13-19(9-10-22(21)23)6-3-18-4-7-20(8-5-18)14-32-12-11-29-24(32)15-33/h4-5,7-13,23,25,30,33,35H,14-17,28H2,1-2H3,(H,31,34)/t23?,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501763

(CHEMBL4099505)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CCO)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C27H30N4O4/c1-18(33)26(27(34)30-35)29-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-31-14-13-28-25(31)12-15-32/h3-4,6-8,10,13-14,16,18,24,26,29,32-33,35H,9,11-12,15,17H2,1H3,(H,30,34)/t18-,24+,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501780

(CHEMBL4083988)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H34N4O3/c1-27(2,28)25(26(32)30-33)29-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-31-13-15-34-16-14-31/h4-5,7-9,11,17,24-25,29,33H,10,12-16,18,28H2,1-2H3,(H,30,32)/t24-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501779

(CHEMBL4102207)Show SMILES C[C@@H](O)[C@H](NC1COCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O5/c1-18(30)25(26(31)28-32)27-24-17-34-16-22-14-20(8-9-23(22)24)5-2-19-3-6-21(7-4-19)15-29-10-12-33-13-11-29/h3-4,6-9,14,18,24-25,27,30,32H,10-13,15-17H2,1H3,(H,28,31)/t18-,24?,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501755

(CHEMBL4077947)Show SMILES C[C@@H](O)[C@H](NC1CCOc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O5/c1-18(30)25(26(31)28-32)27-23-10-13-34-24-16-20(8-9-22(23)24)5-2-19-3-6-21(7-4-19)17-29-11-14-33-15-12-29/h3-4,6-9,16,18,23,25,27,30,32H,10-15,17H2,1H3,(H,28,31)/t18-,23?,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501760
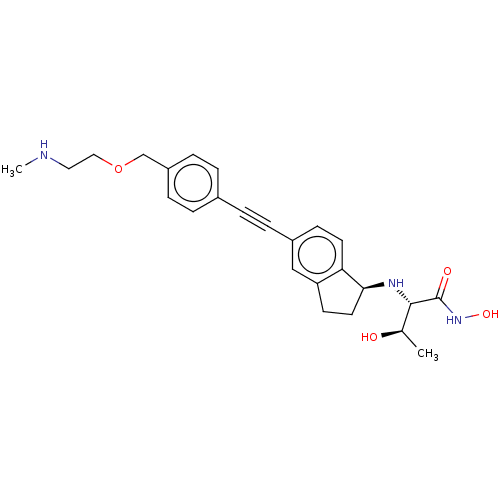
(CHEMBL4078871)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(COCCNC)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C25H31N3O4/c1-17(29)24(25(30)28-31)27-23-12-10-21-15-19(9-11-22(21)23)6-3-18-4-7-20(8-5-18)16-32-14-13-26-2/h4-5,7-9,11,15,17,23-24,26-27,29,31H,10,12-14,16H2,1-2H3,(H,28,30)/t17-,23+,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501786
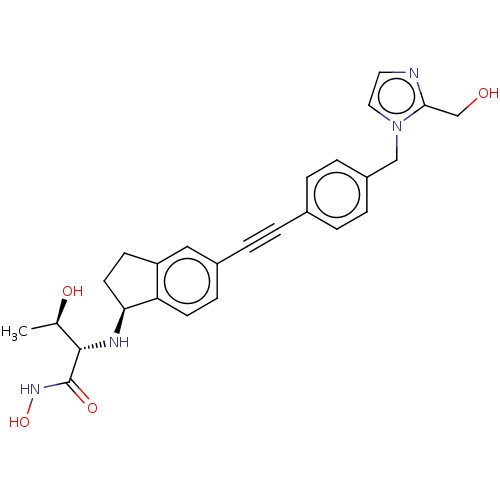
(CHEMBL4089353)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CO)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H28N4O4/c1-17(32)25(26(33)29-34)28-23-11-9-21-14-19(8-10-22(21)23)5-2-18-3-6-20(7-4-18)15-30-13-12-27-24(30)16-31/h3-4,6-8,10,12-14,17,23,25,28,31-32,34H,9,11,15-16H2,1H3,(H,29,33)/t17-,23+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501775
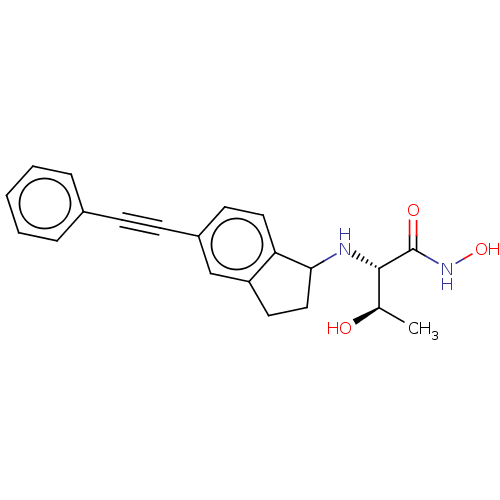
(CHEMBL4077504)Show SMILES C[C@@H](O)[C@H](NC1CCc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O3/c1-14(24)20(21(25)23-26)22-19-12-10-17-13-16(9-11-18(17)19)8-7-15-5-3-2-4-6-15/h2-6,9,11,13-14,19-20,22,24,26H,10,12H2,1H3,(H,23,25)/t14-,19?,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501783

(CHEMBL4064896)Show SMILES [H][C@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501766

(CHEMBL4070896)Show SMILES CC(C)(N)[C@H](NC1COCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H34N4O4/c1-27(2,28)25(26(32)30-33)29-24-18-35-17-22-15-20(9-10-23(22)24)6-3-19-4-7-21(8-5-19)16-31-11-13-34-14-12-31/h4-5,7-10,15,24-25,29,33H,11-14,16-18,28H2,1-2H3,(H,30,32)/t24?,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501758
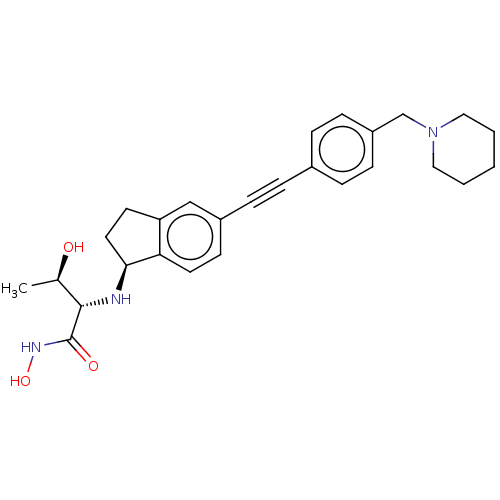
(CHEMBL4104633)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCCCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C27H33N3O3/c1-19(31)26(27(32)29-33)28-25-14-12-23-17-21(11-13-24(23)25)8-5-20-6-9-22(10-7-20)18-30-15-3-2-4-16-30/h6-7,9-11,13,17,19,25-26,28,31,33H,2-4,12,14-16,18H2,1H3,(H,29,32)/t19-,25+,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501758

(CHEMBL4104633)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCCCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C27H33N3O3/c1-19(31)26(27(32)29-33)28-25-14-12-23-17-21(11-13-24(23)25)8-5-20-6-9-22(10-7-20)18-30-15-3-2-4-16-30/h6-7,9-11,13,17,19,25-26,28,31,33H,2-4,12,14-16,18H2,1H3,(H,29,32)/t19-,25+,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501777
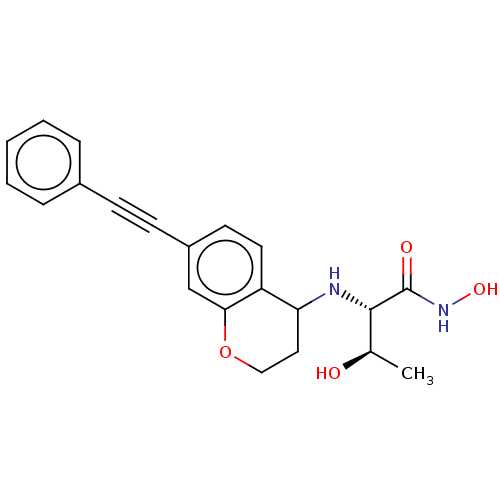
(CHEMBL4070767)Show SMILES C[C@@H](O)[C@H](NC1CCOc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O4/c1-14(24)20(21(25)23-26)22-18-11-12-27-19-13-16(9-10-17(18)19)8-7-15-5-3-2-4-6-15/h2-6,9-10,13-14,18,20,22,24,26H,11-12H2,1H3,(H,23,25)/t14-,18?,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501777

(CHEMBL4070767)Show SMILES C[C@@H](O)[C@H](NC1CCOc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O4/c1-14(24)20(21(25)23-26)22-18-11-12-27-19-13-16(9-10-17(18)19)8-7-15-5-3-2-4-6-15/h2-6,9-10,13-14,18,20,22,24,26H,11-12H2,1H3,(H,23,25)/t14-,18?,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501765
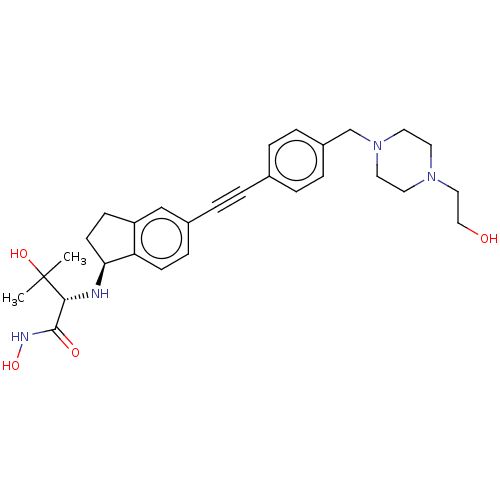
(CHEMBL4062274)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCN(CCO)CC2)cc1)N[C@H](C(=O)NO)C(C)(C)O |r| Show InChI InChI=1S/C29H38N4O4/c1-29(2,36)27(28(35)31-37)30-26-12-10-24-19-22(9-11-25(24)26)6-3-21-4-7-23(8-5-21)20-33-15-13-32(14-16-33)17-18-34/h4-5,7-9,11,19,26-27,30,34,36-37H,10,12-18,20H2,1-2H3,(H,31,35)/t26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501778

(CHEMBL4098674)Show SMILES C[C@@H](O)[C@H](NC1COCc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O4/c1-14(24)20(21(25)23-26)22-19-13-27-12-17-11-16(9-10-18(17)19)8-7-15-5-3-2-4-6-15/h2-6,9-11,14,19-20,22,24,26H,12-13H2,1H3,(H,23,25)/t14-,19?,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501784
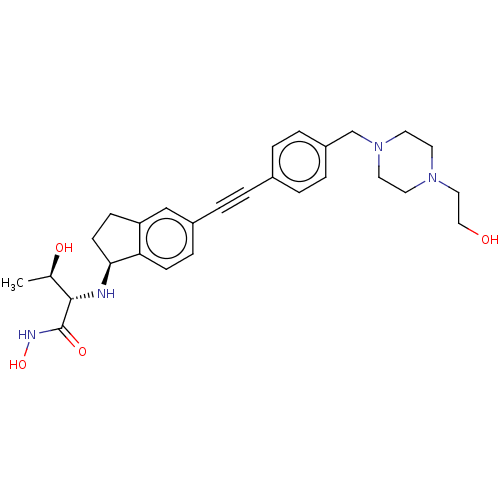
(CHEMBL4086726)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCN(CCO)CC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C28H36N4O4/c1-20(34)27(28(35)30-36)29-26-11-9-24-18-22(8-10-25(24)26)5-2-21-3-6-23(7-4-21)19-32-14-12-31(13-15-32)16-17-33/h3-4,6-8,10,18,20,26-27,29,33-34,36H,9,11-17,19H2,1H3,(H,30,35)/t20-,26+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501768

(CHEMBL4102818)Show SMILES C[C@@H](O)[C@H](NC1CCN(C)c2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H25N3O3/c1-15(26)21(22(27)24-28)23-19-12-13-25(2)20-14-17(10-11-18(19)20)9-8-16-6-4-3-5-7-16/h3-7,10-11,14-15,19,21,23,26,28H,12-13H2,1-2H3,(H,24,27)/t15-,19?,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501760

(CHEMBL4078871)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(COCCNC)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C25H31N3O4/c1-17(29)24(25(30)28-31)27-23-12-10-21-15-19(9-11-22(21)23)6-3-18-4-7-20(8-5-18)16-32-14-13-26-2/h4-5,7-9,11,15,17,23-24,26-27,29,31H,10,12-14,16H2,1-2H3,(H,28,30)/t17-,23+,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50501756

(CHEMBL4066982)Show SMILES C[C@@H](O)[C@H](NC1CCN(C)c2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H34N4O4/c1-19(32)26(27(33)29-34)28-24-11-12-30(2)25-17-21(9-10-23(24)25)6-3-20-4-7-22(8-5-20)18-31-13-15-35-16-14-31/h4-5,7-10,17,19,24,26,28,32,34H,11-16,18H2,1-2H3,(H,29,33)/t19-,24?,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Pseudomonas aeruginosa isolate 44 ATCC 27853 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data