

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210] (Homo sapiens (Human)) | BDBM494877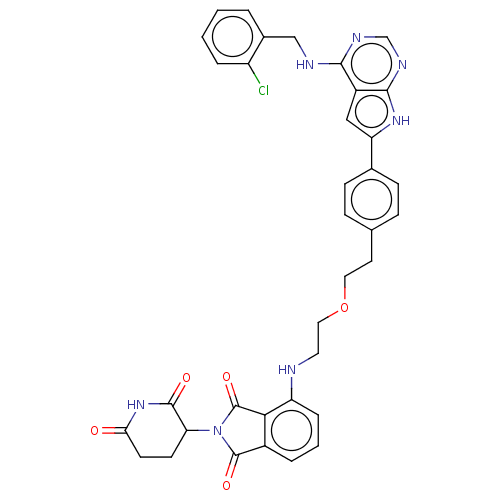 (US10994015, Example 11) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [L858R,T790M,C797S] (Homo sapiens (Human)) | BDBM495017 (US10994015, Example 313 | US10994015, Example 314) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [L858R,T790M,C797S] (Homo sapiens (Human)) | BDBM495012 (US10994015, Example 308) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [T790M,L858R] (Homo sapiens (Human)) | BDBM495017 (US10994015, Example 313 | US10994015, Example 314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210,T790M] (Homo sapiens (Human)) | BDBM495019 (US10994015, Example 319) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [L858R,T790M,C797S] (Homo sapiens (Human)) | BDBM495016 (US10994015, Example 312) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [L858R] (Homo sapiens (Human)) | BDBM494877 (US10994015, Example 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [T790M,L858R] (Homo sapiens (Human)) | BDBM495016 (US10994015, Example 312) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [L858R,T790M,C797S] (Homo sapiens (Human)) | BDBM495019 (US10994015, Example 319) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [T790M,L858R] (Homo sapiens (Human)) | BDBM495012 (US10994015, Example 308) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210] (Homo sapiens (Human)) | BDBM494873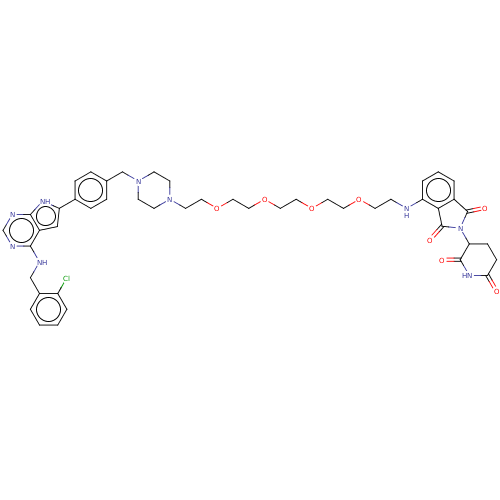 (US10994015, Example 7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [L858R,T790M,C797S] (Homo sapiens (Human)) | BDBM495009 (US10994015, Example 305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210] (Homo sapiens (Human)) | BDBM495019 (US10994015, Example 319) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM50459898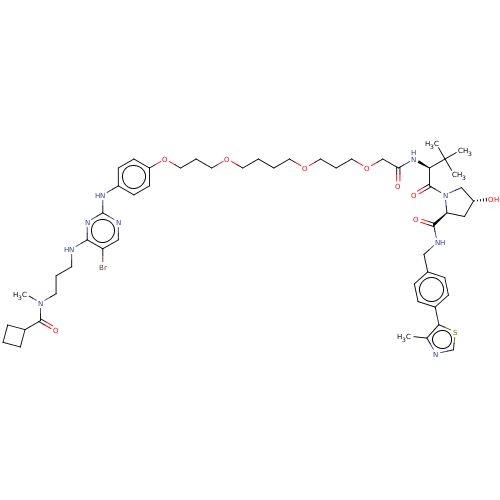 (CHEMBL4227907) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Arvinas LLC Curated by ChEMBL | Assay Description Inhibition of TBK1 (unknown origin) | J Med Chem 61: 583-598 (2018) Article DOI: 10.1021/acs.jmedchem.7b00635 BindingDB Entry DOI: 10.7270/Q2GM89ZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM495017 (US10994015, Example 313 | US10994015, Example 314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210,T790M] (Homo sapiens (Human)) | BDBM495017 (US10994015, Example 313 | US10994015, Example 314) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210] (Homo sapiens (Human)) | BDBM494874 (4-((17-(4-(4-(4-((2-chlorobenzyl)amino)-7H-pyrrolo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210] (Homo sapiens (Human)) | BDBM494875 (US10994015, Example 9) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210] (Homo sapiens (Human)) | BDBM494867 ((2S,4R)-1-((S)-2-(tert-butyl)-14-(4-(4-(4-((2-chlo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210,T790M] (Homo sapiens (Human)) | BDBM495012 (US10994015, Example 308) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [T790M,L858R] (Homo sapiens (Human)) | BDBM495019 (US10994015, Example 319) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210] (Homo sapiens (Human)) | BDBM494872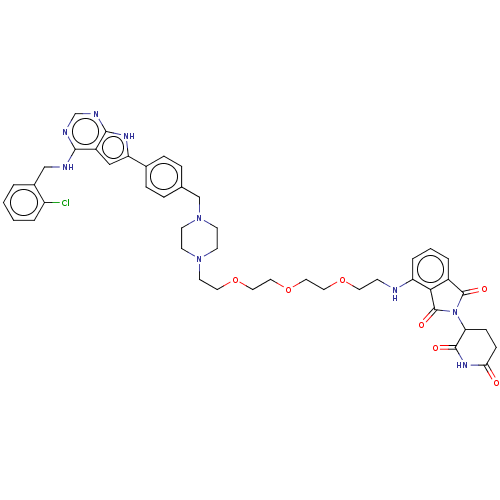 (US10994015, Example 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM485824 (US10946017, Compound 23) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC. US Patent | US Patent US10946017 (2021) BindingDB Entry DOI: 10.7270/Q21N848R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210] (Homo sapiens (Human)) | BDBM494868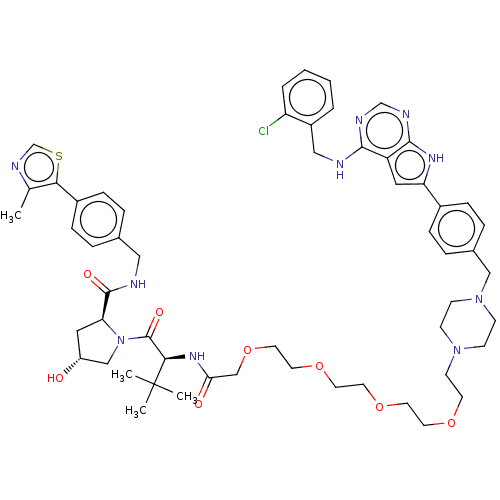 (US10994015, Example 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50438224 (CHEMBL2407759) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP | Bioorg Med Chem Lett 23: 4517-22 (2013) Article DOI: 10.1016/j.bmcl.2013.06.053 BindingDB Entry DOI: 10.7270/Q2ZK5J2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210] (Homo sapiens (Human)) | BDBM494871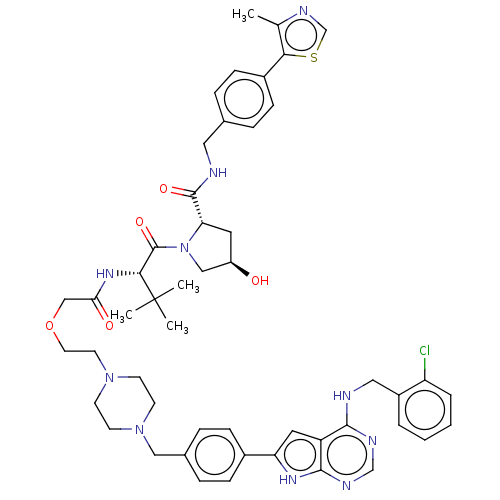 (US10994015, Example 5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210] (Homo sapiens (Human)) | BDBM494870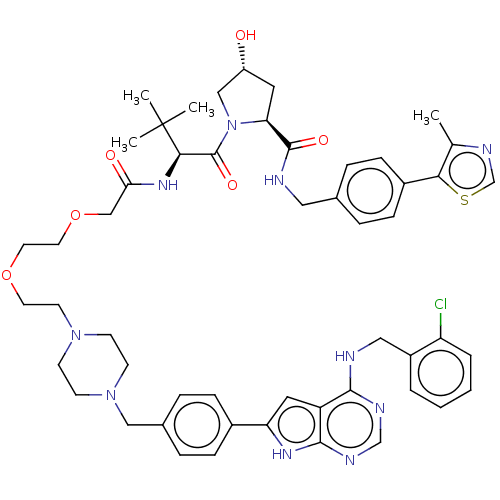 (US10994015, Example 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM495019 (US10994015, Example 319) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM494926 (US10994015, Example 48) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210,T790M] (Homo sapiens (Human)) | BDBM495016 (US10994015, Example 312) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [L858R] (Homo sapiens (Human)) | BDBM494875 (US10994015, Example 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210] (Homo sapiens (Human)) | BDBM494869 (US10994015, Example 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM495013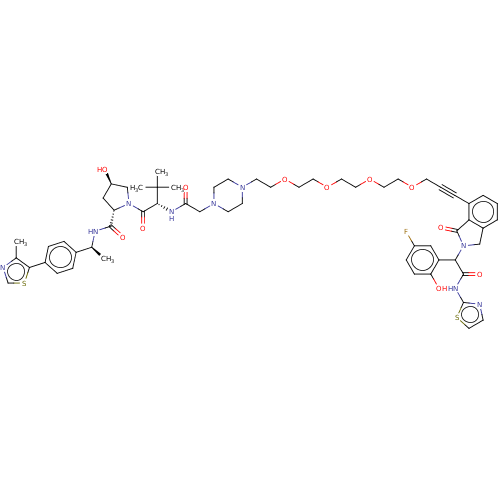 (US10994015, Example 309) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-745,747-749,751-1210] (Homo sapiens (Human)) | BDBM495017 (US10994015, Example 313 | US10994015, Example 314) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [L858R] (Homo sapiens (Human)) | BDBM494870 (US10994015, Example 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM494877 (US10994015, Example 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM50459898 (CHEMBL4227907) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Arvinas LLC Curated by ChEMBL | Assay Description Inhibition of IKKepsilon (unknown origin) | J Med Chem 61: 583-598 (2018) Article DOI: 10.1021/acs.jmedchem.7b00635 BindingDB Entry DOI: 10.7270/Q2GM89ZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50438335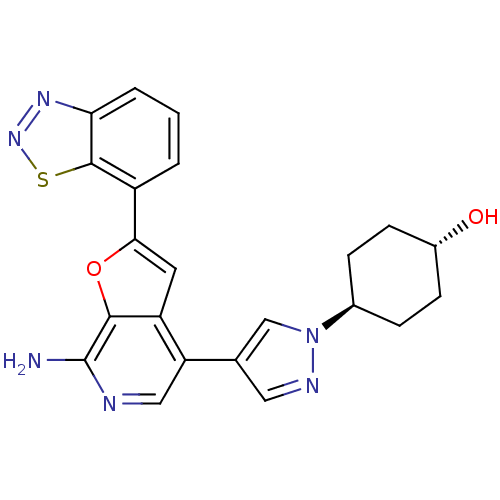 (CHEMBL2408610) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of TAK1-TAB1 (unknown origin) by alphascreen assay in presence of ATP | Bioorg Med Chem Lett 23: 4511-6 (2013) Article DOI: 10.1016/j.bmcl.2013.06.054 BindingDB Entry DOI: 10.7270/Q2FN17MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [T790M,L858R] (Homo sapiens (Human)) | BDBM495009 (US10994015, Example 305) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM485818 (US10946017, Compound 20) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC. US Patent | US Patent US10946017 (2021) BindingDB Entry DOI: 10.7270/Q21N848R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7 (Homo sapiens (Human)) | BDBM50438223 (CHEMBL2407758) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation | Bioorg Med Chem Lett 23: 4517-22 (2013) Article DOI: 10.1016/j.bmcl.2013.06.053 BindingDB Entry DOI: 10.7270/Q2ZK5J2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50421257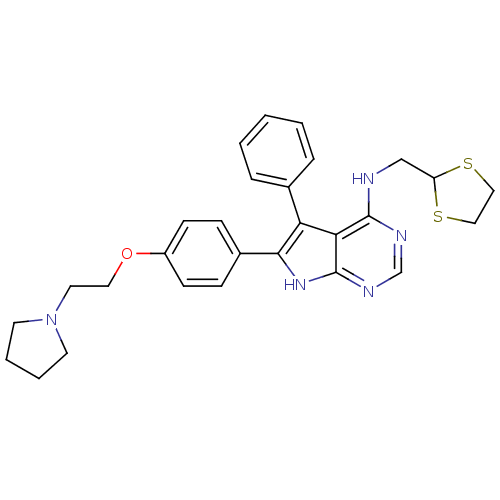 (CHEMBL2087875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, LLC Curated by ChEMBL | Assay Description Inhibition of ACK1 (unknown origin) by cellular mechanistic assay | Bioorg Med Chem Lett 23: 979-84 (2013) Article DOI: 10.1016/j.bmcl.2012.12.042 BindingDB Entry DOI: 10.7270/Q2348MQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [L858R] (Homo sapiens (Human)) | BDBM494874 (4-((17-(4-(4-(4-((2-chlorobenzyl)amino)-7H-pyrrolo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [L858R] (Homo sapiens (Human)) | BDBM494871 (US10994015, Example 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM494927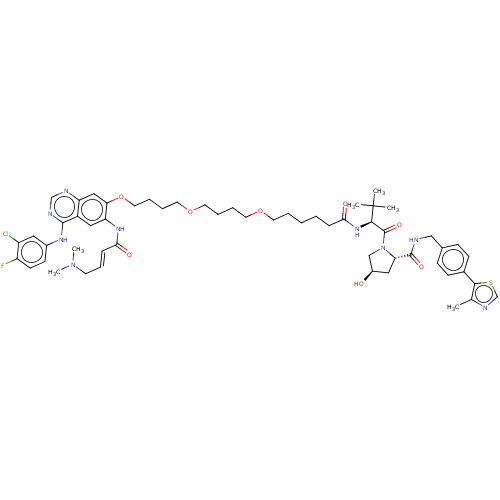 ((2S,4R)-1-((S)-2-(6-(4-(4-((4-((3-chloro-4-fluorop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM485844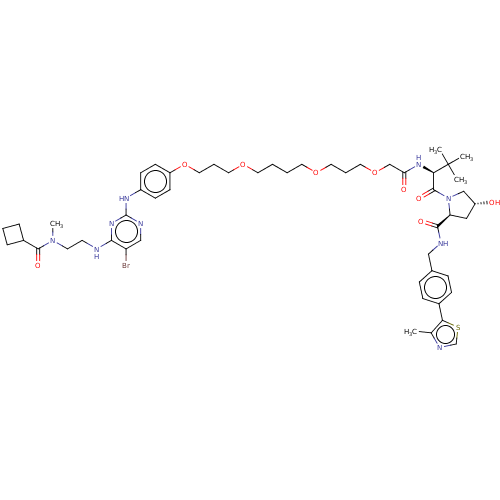 (US10946017, Compound 11DC50:) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC. US Patent | US Patent US10946017 (2021) BindingDB Entry DOI: 10.7270/Q21N848R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM494956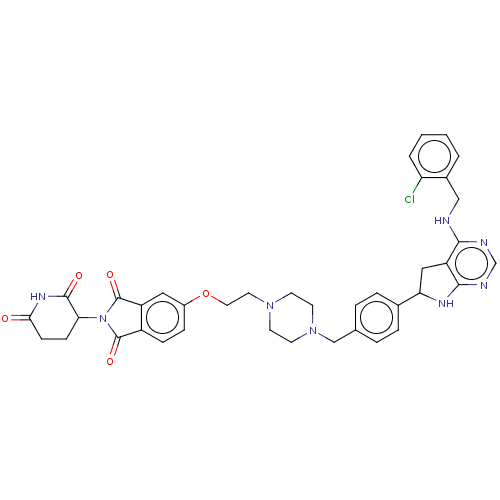 (US10994015, Example 101) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [L858R] (Homo sapiens (Human)) | BDBM494868 (US10994015, Example 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ARVINAS OPERATIONS, INC.; YALE UNIVERSITY US Patent | Assay Description All compounds and PROTACs were serially diluted in three-fold increments using 100% DMSO, followed by an intermediate 10-fold dilution using Buffer A... | US Patent US10994015 (2021) BindingDB Entry DOI: 10.7270/Q2NS0Z1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7 (Homo sapiens (Human)) | BDBM50438224 (CHEMBL2407759) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylation | Bioorg Med Chem Lett 23: 4517-22 (2013) Article DOI: 10.1016/j.bmcl.2013.06.053 BindingDB Entry DOI: 10.7270/Q2ZK5J2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM50438223 (CHEMBL2407758) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals LLC Curated by ChEMBL | Assay Description Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATP | Bioorg Med Chem Lett 23: 4517-22 (2013) Article DOI: 10.1016/j.bmcl.2013.06.053 BindingDB Entry DOI: 10.7270/Q2ZK5J2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1730 total ) | Next | Last >> |