 Found 44 hits with Last Name = 'crowder' and Initial = 'mw'
Found 44 hits with Last Name = 'crowder' and Initial = 'mw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191983
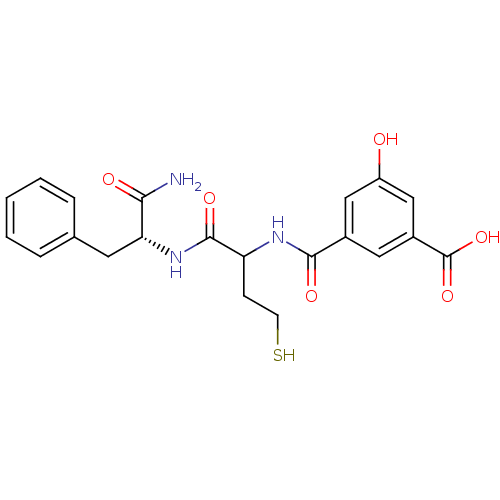
(3-((1-((R)-1-amino-1-oxo-3-phenylpropan-2-ylamino)...)Show SMILES NC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1cc(O)cc(c1)C(O)=O Show InChI InChI=1S/C21H23N3O6S/c22-18(26)17(8-12-4-2-1-3-5-12)24-20(28)16(6-7-31)23-19(27)13-9-14(21(29)30)11-15(25)10-13/h1-5,9-11,16-17,25,31H,6-8H2,(H2,22,26)(H,23,27)(H,24,28)(H,29,30)/t16?,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191983

(3-((1-((R)-1-amino-1-oxo-3-phenylpropan-2-ylamino)...)Show SMILES NC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1cc(O)cc(c1)C(O)=O Show InChI InChI=1S/C21H23N3O6S/c22-18(26)17(8-12-4-2-1-3-5-12)24-20(28)16(6-7-31)23-19(27)13-9-14(21(29)30)11-15(25)10-13/h1-5,9-11,16-17,25,31H,6-8H2,(H2,22,26)(H,23,27)(H,24,28)(H,29,30)/t16?,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191986
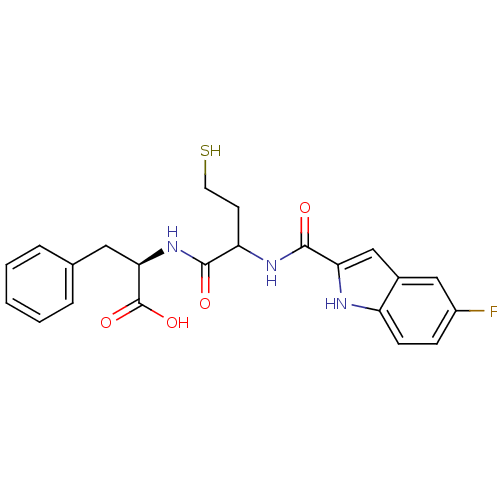
((2R)-2-(2-(5-fluoro-1H-indole-2-carboxamido)-4-mer...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1cc2cc(F)ccc2[nH]1 Show InChI InChI=1S/C22H22FN3O4S/c23-15-6-7-16-14(11-15)12-18(24-16)21(28)25-17(8-9-31)20(27)26-19(22(29)30)10-13-4-2-1-3-5-13/h1-7,11-12,17,19,24,31H,8-10H2,(H,25,28)(H,26,27)(H,29,30)/t17?,19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191996
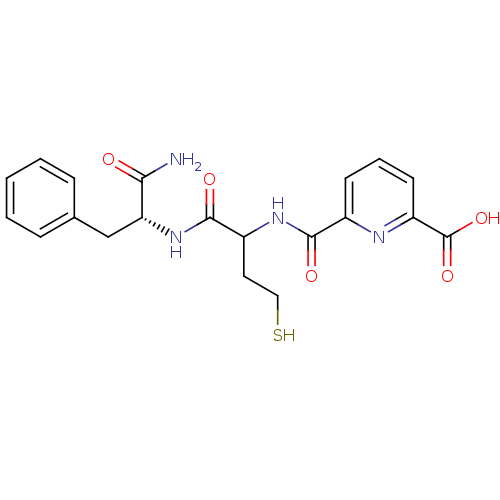
(6-((1-((R)-1-amino-1-oxo-3-phenylpropan-2-ylamino)...)Show SMILES NC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1cccc(n1)C(O)=O Show InChI InChI=1S/C20H22N4O5S/c21-17(25)16(11-12-5-2-1-3-6-12)24-19(27)14(9-10-30)23-18(26)13-7-4-8-15(22-13)20(28)29/h1-8,14,16,30H,9-11H2,(H2,21,25)(H,23,26)(H,24,27)(H,28,29)/t14?,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191987

(6-((1-((R)-1-carboxy-2-phenylethylamino)-4-mercapt...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1cccc(n1)C(O)=O Show InChI InChI=1S/C20H21N3O6S/c24-17(13-7-4-8-15(21-13)19(26)27)22-14(9-10-30)18(25)23-16(20(28)29)11-12-5-2-1-3-6-12/h1-8,14,16,30H,9-11H2,(H,22,24)(H,23,25)(H,26,27)(H,28,29)/t14?,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191987

(6-((1-((R)-1-carboxy-2-phenylethylamino)-4-mercapt...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1cccc(n1)C(O)=O Show InChI InChI=1S/C20H21N3O6S/c24-17(13-7-4-8-15(21-13)19(26)27)22-14(9-10-30)18(25)23-16(20(28)29)11-12-5-2-1-3-6-12/h1-8,14,16,30H,9-11H2,(H,22,24)(H,23,25)(H,26,27)(H,28,29)/t14?,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191983

(3-((1-((R)-1-amino-1-oxo-3-phenylpropan-2-ylamino)...)Show SMILES NC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1cc(O)cc(c1)C(O)=O Show InChI InChI=1S/C21H23N3O6S/c22-18(26)17(8-12-4-2-1-3-5-12)24-20(28)16(6-7-31)23-19(27)13-9-14(21(29)30)11-15(25)10-13/h1-5,9-11,16-17,25,31H,6-8H2,(H2,22,26)(H,23,27)(H,24,28)(H,29,30)/t16?,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50192000

(3-((1-((R)-1-carboxy-2-phenylethylamino)-4-mercapt...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1cc(O)cc(c1)C(O)=O Show InChI InChI=1S/C21H22N2O7S/c24-15-10-13(9-14(11-15)20(27)28)18(25)22-16(6-7-31)19(26)23-17(21(29)30)8-12-4-2-1-3-5-12/h1-5,9-11,16-17,24,31H,6-8H2,(H,22,25)(H,23,26)(H,27,28)(H,29,30)/t16?,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191991

((2R)-2-(4-mercapto-2-(7-nitro-1H-indole-2-carboxam...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1cc2cccc([N+]([O-])=O)c2[nH]1 Show InChI InChI=1S/C22H22N4O6S/c27-20(25-17(22(29)30)11-13-5-2-1-3-6-13)15(9-10-33)24-21(28)16-12-14-7-4-8-18(26(31)32)19(14)23-16/h1-8,12,15,17,23,33H,9-11H2,(H,24,28)(H,25,27)(H,29,30)/t15?,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191982

((2R)-2-(4-mercapto-2-(2-(2-nitrophenyl)furan-5-car...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1ccc(o1)-c1ccccc1[N+]([O-])=O Show InChI InChI=1S/C24H23N3O7S/c28-22(26-18(24(30)31)14-15-6-2-1-3-7-15)17(12-13-35)25-23(29)21-11-10-20(34-21)16-8-4-5-9-19(16)27(32)33/h1-11,17-18,35H,12-14H2,(H,25,29)(H,26,28)(H,30,31)/t17?,18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50192002

((2R)-2-(2-(4-(benzyloxy)-3,5-dimethylbenzamido)-4-...)Show SMILES Cc1cc(cc(C)c1OCc1ccccc1)C(=O)NC(CCS)C(=O)N[C@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C29H32N2O5S/c1-19-15-23(16-20(2)26(19)36-18-22-11-7-4-8-12-22)27(32)30-24(13-14-37)28(33)31-25(29(34)35)17-21-9-5-3-6-10-21/h3-12,15-16,24-25,37H,13-14,17-18H2,1-2H3,(H,30,32)(H,31,33)(H,34,35)/t24?,25-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191992
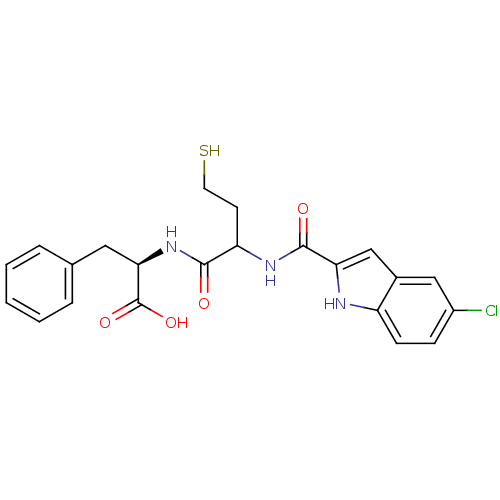
((2R)-2-(2-(5-chloro-1H-indole-2-carboxamido)-4-mer...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C22H22ClN3O4S/c23-15-6-7-16-14(11-15)12-18(24-16)21(28)25-17(8-9-31)20(27)26-19(22(29)30)10-13-4-2-1-3-5-13/h1-7,11-12,17,19,24,31H,8-10H2,(H,25,28)(H,26,27)(H,29,30)/t17?,19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191998
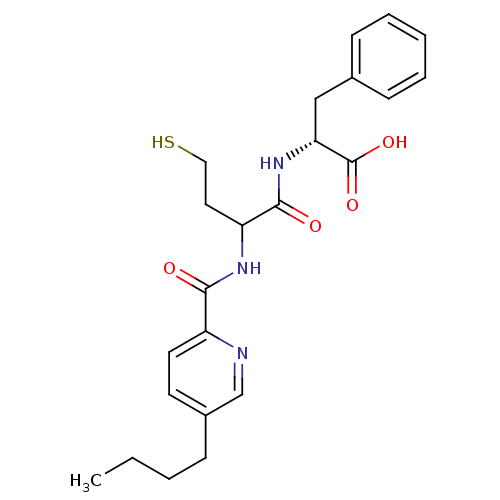
((2R)-2-(2-(3-butylpicolinamido)-4-mercaptobutanami...)Show SMILES CCCCc1ccc(nc1)C(=O)NC(CCS)C(=O)N[C@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C23H29N3O4S/c1-2-3-7-17-10-11-18(24-15-17)21(27)25-19(12-13-31)22(28)26-20(23(29)30)14-16-8-5-4-6-9-16/h4-6,8-11,15,19-20,31H,2-3,7,12-14H2,1H3,(H,25,27)(H,26,28)(H,29,30)/t19?,20-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50192003
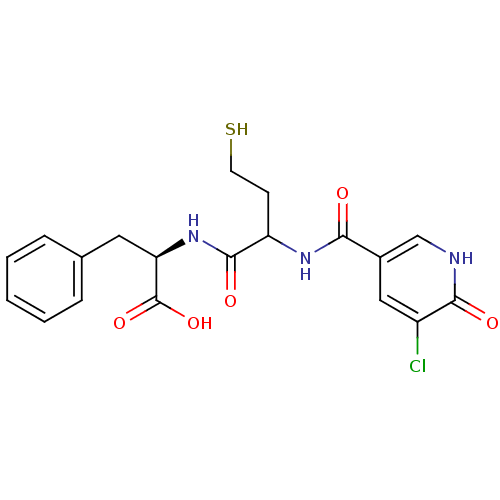
((2R)-2-(2-(3-chloro-2-hydroxynicotinamido)-4-merca...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1c[nH]c(=O)c(Cl)c1 Show InChI InChI=1S/C19H20ClN3O5S/c20-13-9-12(10-21-17(13)25)16(24)22-14(6-7-29)18(26)23-15(19(27)28)8-11-4-2-1-3-5-11/h1-5,9-10,14-15,29H,6-8H2,(H,21,25)(H,22,24)(H,23,26)(H,27,28)/t14?,15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191997
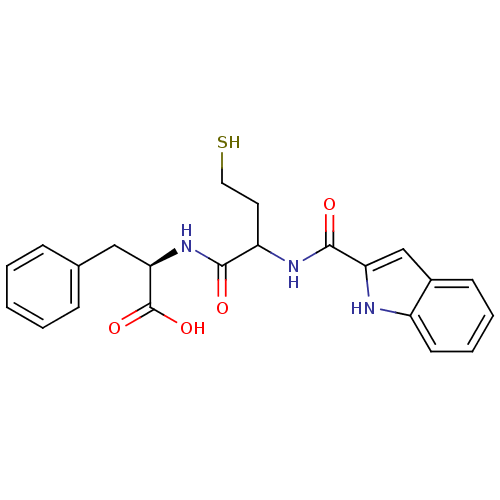
((2R)-2-(2-(1H-indole-2-carboxamido)-4-mercaptobuta...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C22H23N3O4S/c26-20(25-19(22(28)29)12-14-6-2-1-3-7-14)17(10-11-30)24-21(27)18-13-15-8-4-5-9-16(15)23-18/h1-9,13,17,19,23,30H,10-12H2,(H,24,27)(H,25,26)(H,28,29)/t17?,19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191989

((2R)-2-(4-mercapto-2-(2-(naphthalen-1-yloxy)acetam...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)COc1cccc2ccccc12 Show InChI InChI=1S/C25H26N2O5S/c28-23(16-32-22-12-6-10-18-9-4-5-11-19(18)22)26-20(13-14-33)24(29)27-21(25(30)31)15-17-7-2-1-3-8-17/h1-12,20-21,33H,13-16H2,(H,26,28)(H,27,29)(H,30,31)/t20?,21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191984
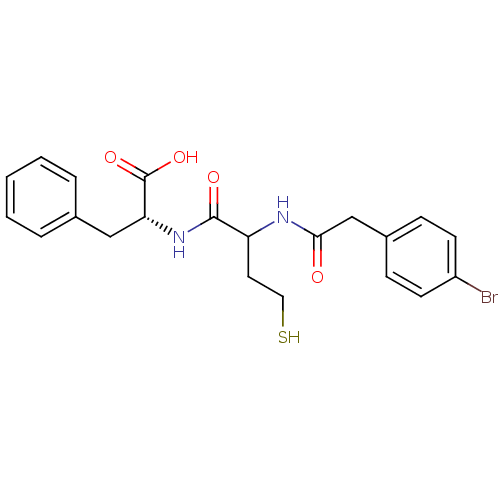
((2R)-2-(2-(2-(4-bromophenyl)acetamido)-4-mercaptob...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)Cc1ccc(Br)cc1 Show InChI InChI=1S/C21H23BrN2O4S/c22-16-8-6-15(7-9-16)13-19(25)23-17(10-11-29)20(26)24-18(21(27)28)12-14-4-2-1-3-5-14/h1-9,17-18,29H,10-13H2,(H,23,25)(H,24,26)(H,27,28)/t17?,18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50192001
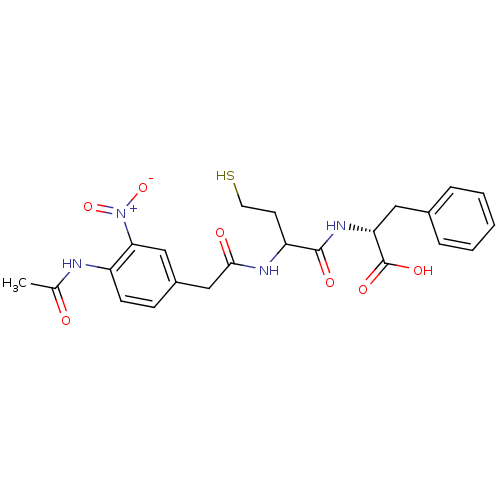
((2R)-2-(2-(2-(4-acetamido-3-nitrophenyl)acetamido)...)Show SMILES CC(=O)Nc1ccc(CC(=O)NC(CCS)C(=O)N[C@H](Cc2ccccc2)C(O)=O)cc1[N+]([O-])=O Show InChI InChI=1S/C23H26N4O7S/c1-14(28)24-17-8-7-16(12-20(17)27(33)34)13-21(29)25-18(9-10-35)22(30)26-19(23(31)32)11-15-5-3-2-4-6-15/h2-8,12,18-19,35H,9-11,13H2,1H3,(H,24,28)(H,25,29)(H,26,30)(H,31,32)/t18?,19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191985

((2R)-2-(4-mercapto-2-(3-(trimethylsilyl)propanamid...)Show SMILES C[Si](C)(C)CCC(=O)NC(CCS)C(=O)N[C@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C19H30N2O4SSi/c1-27(2,3)12-10-17(22)20-15(9-11-26)18(23)21-16(19(24)25)13-14-7-5-4-6-8-14/h4-8,15-16,26H,9-13H2,1-3H3,(H,20,22)(H,21,23)(H,24,25)/t15?,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191988

((2R)-2-(2-(4-hydroxy-3,5-dimethylbenzamido)-4-merc...)Show SMILES Cc1cc(cc(C)c1O)C(=O)NC(CCS)C(=O)N[C@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C22H26N2O5S/c1-13-10-16(11-14(2)19(13)25)20(26)23-17(8-9-30)21(27)24-18(22(28)29)12-15-6-4-3-5-7-15/h3-7,10-11,17-18,25,30H,8-9,12H2,1-2H3,(H,23,26)(H,24,27)(H,28,29)/t17?,18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191994

((2R)-2-(2-(5-hydroxy-1H-indole-2-carboxamido)-4-me...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)c1cc2cc(O)ccc2[nH]1 Show InChI InChI=1S/C22H23N3O5S/c26-15-6-7-16-14(11-15)12-18(23-16)21(28)24-17(8-9-31)20(27)25-19(22(29)30)10-13-4-2-1-3-5-13/h1-7,11-12,17,19,23,26,31H,8-10H2,(H,24,28)(H,25,27)(H,29,30)/t17?,19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191995

((2R)-2-(2-(2-(2-chloro-5-nitrophenyl)acetamido)-4-...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)Cc1cc(ccc1Cl)[N+]([O-])=O Show InChI InChI=1S/C21H22ClN3O6S/c22-16-7-6-15(25(30)31)11-14(16)12-19(26)23-17(8-9-32)20(27)24-18(21(28)29)10-13-4-2-1-3-5-13/h1-7,11,17-18,32H,8-10,12H2,(H,23,26)(H,24,27)(H,28,29)/t17?,18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191993

((2R)-2-(2-(3-cyclohexylpropanamido)-4-mercaptobuta...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)CCC1CCCCC1 Show InChI InChI=1S/C22H32N2O4S/c25-20(12-11-16-7-3-1-4-8-16)23-18(13-14-29)21(26)24-19(22(27)28)15-17-9-5-2-6-10-17/h2,5-6,9-10,16,18-19,29H,1,3-4,7-8,11-15H2,(H,23,25)(H,24,26)(H,27,28)/t18?,19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50192004

((2R)-2-(2-(3-butylpicolinamido)-3-mercaptopropanam...)Show SMILES CCCCc1ccc(nc1)C(=O)NC(CS)C(=O)N[C@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C22H27N3O4S/c1-2-3-7-16-10-11-17(23-13-16)20(26)25-19(14-30)21(27)24-18(22(28)29)12-15-8-5-4-6-9-15/h4-6,8-11,13,18-19,30H,2-3,7,12,14H2,1H3,(H,24,27)(H,25,26)(H,28,29)/t18-,19?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Escherichia coli (strain K12)) | BDBM50173997

((1-aminoethyl)(2-carboxy-1-propenyl)phosphinic aci...)Show InChI InChI=1S/C6H12NO4P/c1-4(6(8)9)3-12(10,11)5(2)7/h4,7H,3H2,1-2H3,(H,8,9)(H,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Miami University
Curated by ChEMBL
| Assay Description
Inhibitory activity against aminopeptidase N (PepN) from Escherichia coli |
Bioorg Med Chem Lett 15: 5150-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.055
BindingDB Entry DOI: 10.7270/Q2SB45B8 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191990

((2R)-2-(4-mercapto-2-(2-(3-nitrophenyl)acetamido)b...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CCS)NC(=O)Cc1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H23N3O6S/c25-19(13-15-7-4-8-16(11-15)24(29)30)22-17(9-10-31)20(26)23-18(21(27)28)12-14-5-2-1-3-6-14/h1-8,11,17-18,31H,9-10,12-13H2,(H,22,25)(H,23,26)(H,27,28)/t17?,18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Escherichia coli (strain K12)) | BDBM50173996

((1-aminoethyl)(2-carboxy-1-decyl)phosphinic acid |...)Show InChI InChI=1S/C13H28NO4P/c1-3-4-5-6-7-8-9-12(13(15)16)10-19(17,18)11(2)14/h12,14,17-19H,3-10H2,1-2H3,(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Miami University
Curated by ChEMBL
| Assay Description
Inhibitory activity against aminopeptidase N (PepN) from Escherichia coli |
Bioorg Med Chem Lett 15: 5150-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.055
BindingDB Entry DOI: 10.7270/Q2SB45B8 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase L1 type 3
(Stenotrophomonas maltophilia) | BDBM50191999

((2R)-2-(2-(2-(4-bromophenyl)acetamido)-3-mercaptop...)Show SMILES OC(=O)[C@@H](Cc1ccccc1)NC(=O)C(CS)NC(=O)Cc1ccc(Br)cc1 Show InChI InChI=1S/C20H21BrN2O4S/c21-15-8-6-14(7-9-15)11-18(24)22-17(12-28)19(25)23-16(20(26)27)10-13-4-2-1-3-5-13/h1-9,16-17,28H,10-12H2,(H,22,24)(H,23,25)(H,26,27)/t16-,17?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of Stenotrophomonas maltophilia L1 metallo beta lactamase |
Bioorg Med Chem Lett 16: 5169-75 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.001
BindingDB Entry DOI: 10.7270/Q2WQ03DJ |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Escherichia coli (strain K12)) | BDBM50022572

(3-[(1-Amino-ethyl)-hydroxy-phosphinoyl]-2-methyl-p...)Show InChI InChI=1S/C6H14NO4P/c1-4(6(8)9)3-12(10,11)5(2)7/h4,7,10-12H,3H2,1-2H3,(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Miami University
Curated by ChEMBL
| Assay Description
Inhibitory activity against aminopeptidase N (PepN) from Escherichia coli |
Bioorg Med Chem Lett 15: 5150-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.055
BindingDB Entry DOI: 10.7270/Q2SB45B8 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Escherichia coli (strain K12)) | BDBM50173999
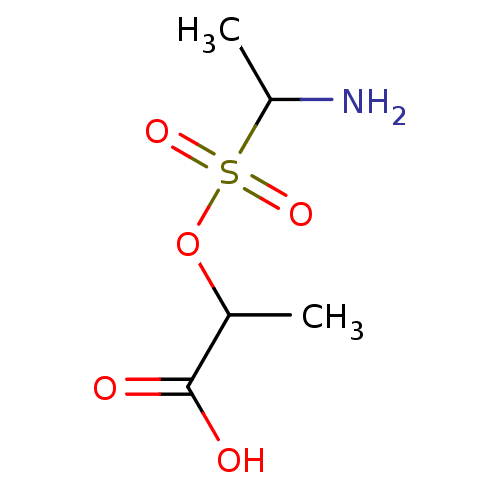
(1-aminoethyl-O-1-carboxyethyl sulfonate | CHEMBL36...)Show InChI InChI=1S/C5H11NO5S/c1-3(5(7)8)11-12(9,10)4(2)6/h3-4H,6H2,1-2H3,(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Miami University
Curated by ChEMBL
| Assay Description
Inhibitory activity against aminopeptidase N (PepN) from Escherichia coli |
Bioorg Med Chem Lett 15: 5150-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.055
BindingDB Entry DOI: 10.7270/Q2SB45B8 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Escherichia coli (strain K12)) | BDBM50173998

(1-aminoethyl-N-1-carboxyethyl sulfonamidate | CHEM...)Show InChI InChI=1S/C5H12N2O4S/c1-3(5(8)9)7-12(10,11)4(2)6/h3-4,7H,6H2,1-2H3,(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Miami University
Curated by ChEMBL
| Assay Description
Inhibitory activity against aminopeptidase N (PepN) from Escherichia coli |
Bioorg Med Chem Lett 15: 5150-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.055
BindingDB Entry DOI: 10.7270/Q2SB45B8 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase VIM-2
(Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM50240870
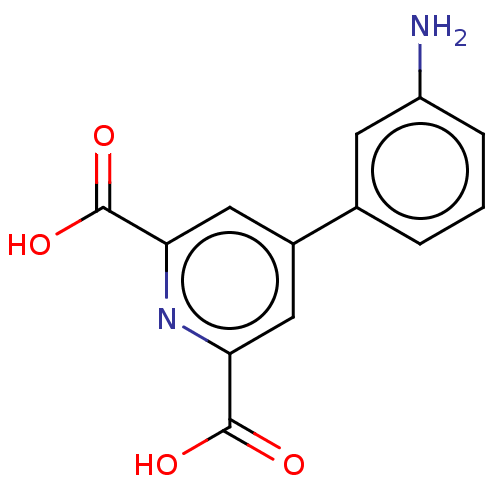
(CHEMBL4068259)Show InChI InChI=1S/C13H10N2O4/c14-9-3-1-2-7(4-9)8-5-10(12(16)17)15-11(6-8)13(18)19/h1-6H,14H2,(H,16,17)(H,18,19) | PDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. |
J Med Chem 60: 7267-7283 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00407
BindingDB Entry DOI: 10.7270/Q2P27185 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Serratia marcescens) | BDBM50240870

(CHEMBL4068259)Show InChI InChI=1S/C13H10N2O4/c14-9-3-1-2-7(4-9)8-5-10(12(16)17)15-11(6-8)13(18)19/h1-6H,14H2,(H,16,17)(H,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Inhibition of Serratia marcescens IMP1 expressed in Escherichia coli BL21(DE3) using chromacef as substrate preincubated for 10 mins followed by subs... |
J Med Chem 60: 7267-7283 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00407
BindingDB Entry DOI: 10.7270/Q2P27185 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase VIM-2
(Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM26116
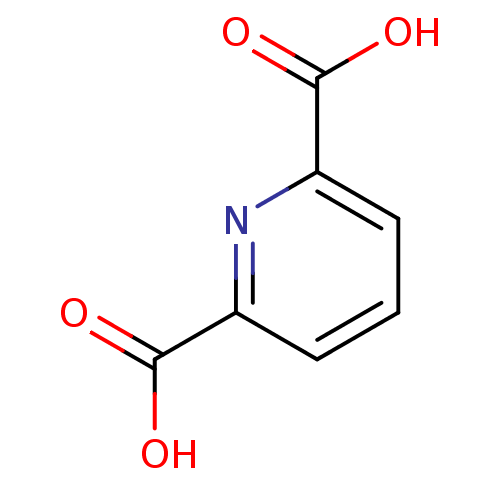
(CHEMBL284104 | Dipicolinate | pyridine carboxylate...)Show InChI InChI=1S/C7H5NO4/c9-6(10)4-2-1-3-5(8-4)7(11)12/h1-3H,(H,9,10)(H,11,12) | PDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa VIM2 expressed in Escherichia coli BL21(DE3) using chromacef as substrate preincubated for 10 mins followed by s... |
J Med Chem 60: 7267-7283 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00407
BindingDB Entry DOI: 10.7270/Q2P27185 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Serratia marcescens) | BDBM26116

(CHEMBL284104 | Dipicolinate | pyridine carboxylate...)Show InChI InChI=1S/C7H5NO4/c9-6(10)4-2-1-3-5(8-4)7(11)12/h1-3H,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Diego
Curated by ChEMBL
| Assay Description
Binding affinity at 5-hydroxytryptamine 1A receptor by the displacement of [3H]8-OH-DPAT in rat brain. |
J Med Chem 60: 7267-7283 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00407
BindingDB Entry DOI: 10.7270/Q2P27185 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50609681
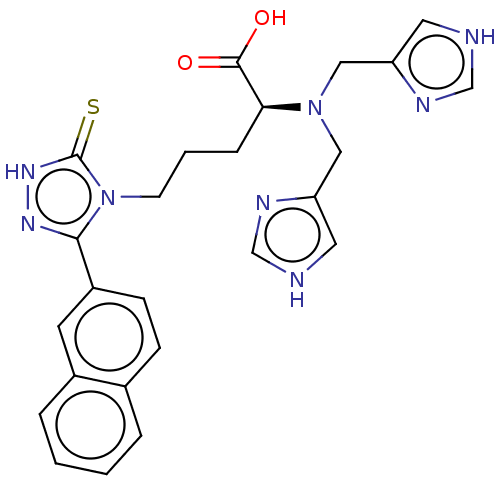
(CHEMBL5271623)Show SMILES OC(=O)[C@H](CCCn1c(n[nH]c1=S)-c1ccc2ccccc2c1)N(Cc1c[nH]cn1)Cc1c[nH]cn1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50609681

(CHEMBL5271623)Show SMILES OC(=O)[C@H](CCCn1c(n[nH]c1=S)-c1ccc2ccccc2c1)N(Cc1c[nH]cn1)Cc1c[nH]cn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
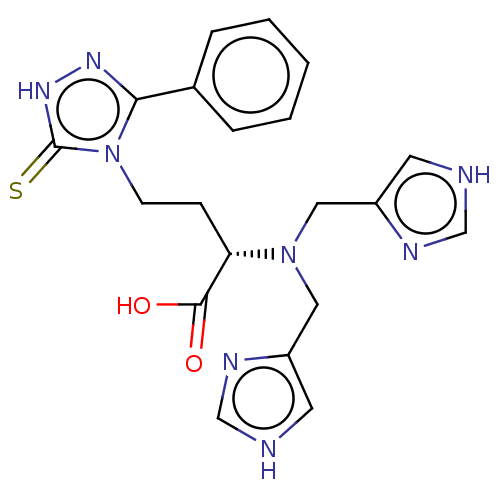
(Homo sapiens (Human)) | BDBM50609678
(CHEMBL5287299)Show SMILES OC(=O)[C@H](CCn1c(n[nH]c1=S)-c1ccccc1)N(Cc1c[nH]cn1)Cc1c[nH]cn1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
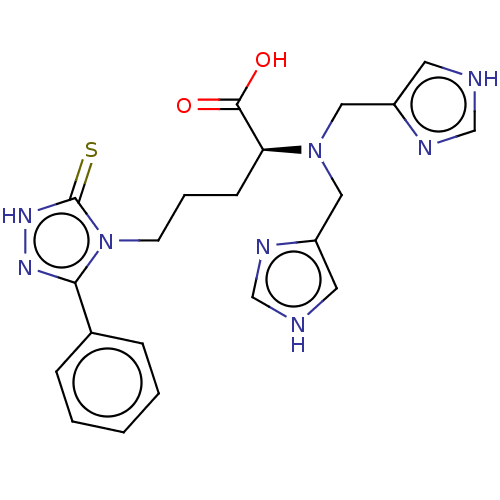
(Homo sapiens (Human)) | BDBM50609677
(CHEMBL5272558)Show SMILES OC(=O)[C@H](CCCn1c(n[nH]c1=S)-c1ccccc1)N(Cc1c[nH]cn1)Cc1c[nH]cn1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50609678

(CHEMBL5287299)Show SMILES OC(=O)[C@H](CCn1c(n[nH]c1=S)-c1ccccc1)N(Cc1c[nH]cn1)Cc1c[nH]cn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50609677

(CHEMBL5272558)Show SMILES OC(=O)[C@H](CCCn1c(n[nH]c1=S)-c1ccccc1)N(Cc1c[nH]cn1)Cc1c[nH]cn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PDB
UniChem
| | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
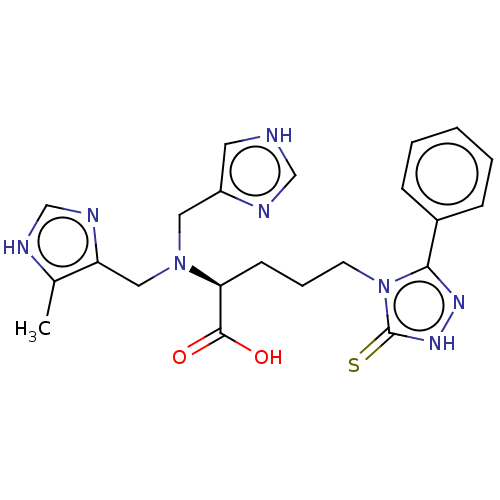
(Homo sapiens (Human)) | BDBM50609680
(CHEMBL5278110)Show SMILES Cc1[nH]cnc1CN(Cc1c[nH]cn1)[C@@H](CCCn1c(n[nH]c1=S)-c1ccccc1)C(O)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50609679
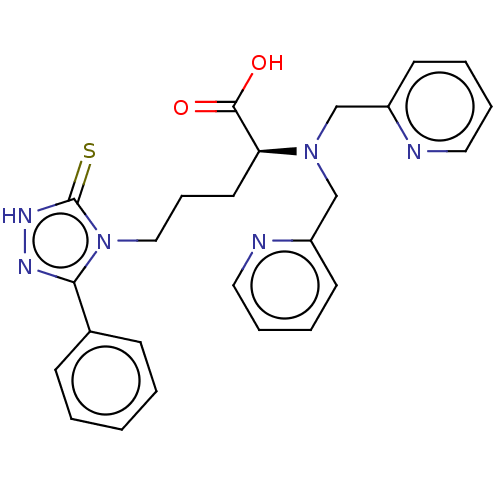
(CHEMBL5280464)Show SMILES OC(=O)[C@H](CCCn1c(n[nH]c1=S)-c1ccccc1)N(Cc1ccccn1)Cc1ccccn1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hydroxyacylglutathione hydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50582857

(CHEMBL5091922) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Glyoxalase-2 assessed as reduction of substrate level using S-D-lactoylglutathione as substrate by spectrophotometric... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113873
BindingDB Entry DOI: 10.7270/Q2B56PM2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data