

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Lactobacillus casei) | BDBM18771 ((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of Thymidylate Synthase in L. casei, | J Med Chem 29: 478-82 (1986) BindingDB Entry DOI: 10.7270/Q2CR5SCZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016343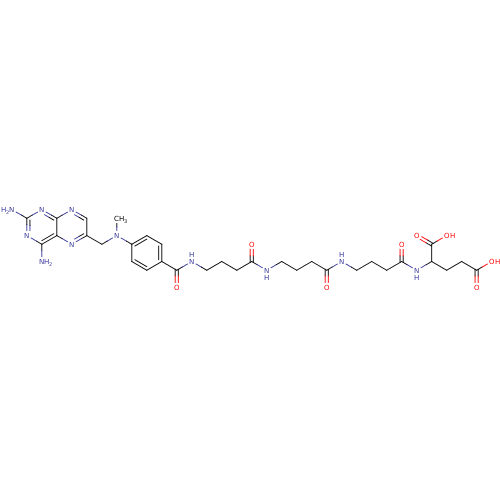 (2-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016340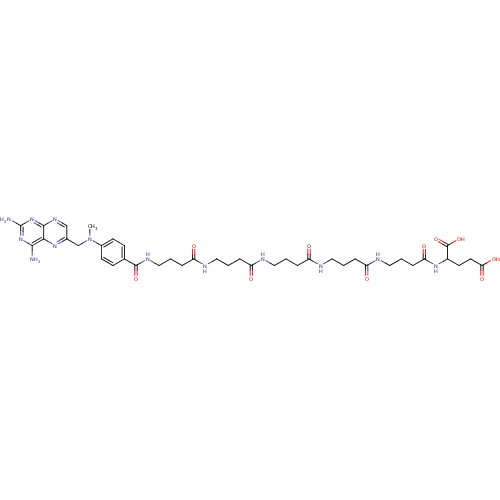 (2-[4-(4-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016339 (2-(4-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016342 (2-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016341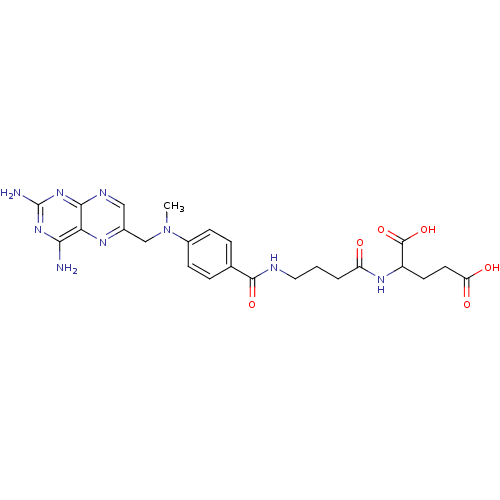 (2-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50028378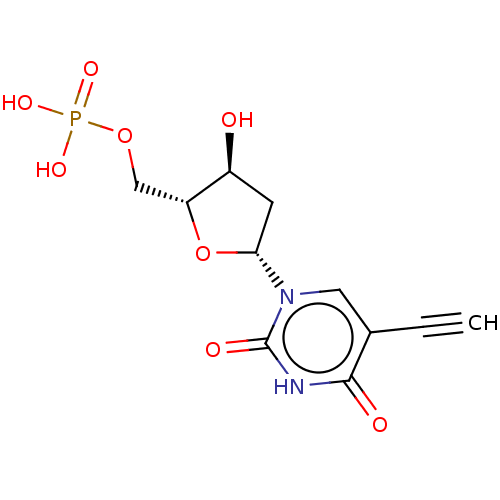 (CHEMBL3143871 | Phosphoric acid mono-[5-(5-ethynyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for inhibition of dTMP synthetase from L. casei | J Med Chem 24: 1537-40 (1982) BindingDB Entry DOI: 10.7270/Q2H1311M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Mus musculus) | BDBM50452155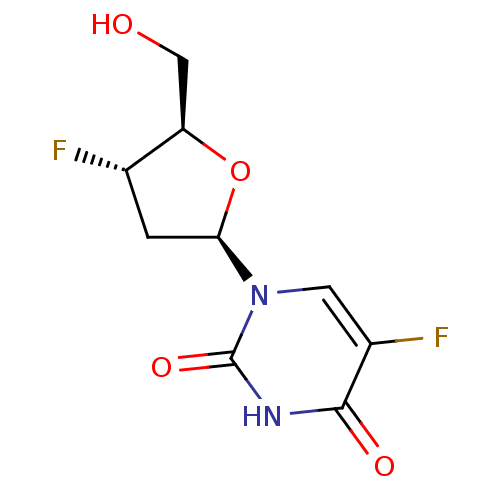 (3''-FFdUrd | CHEMBL1098358) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for non-competitive inhibition of cleavage of Thymidine (dThd) phosphorylase isolated from Lewis lung carcinoma. | J Med Chem 27: 11-4 (1984) BindingDB Entry DOI: 10.7270/Q2J67FXS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit dihydrofolate reductase purified from murine L1210 cells | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016341 (2-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit dihydrofolate reductase purified from murine L1210 cells | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016342 (2-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit dihydrofolate reductase purified from murine L1210 cells | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016343 (2-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit dihydrofolate reductase purified from murine L1210 cells | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016339 (2-(4-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit dihydrofolate reductase purified from murine L1210 cells | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016340 (2-[4-(4-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit dihydrofolate reductase purified from murine L1210 cells | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||