 Found 40 hits with Last Name = 'datta' and Initial = 's'
Found 40 hits with Last Name = 'datta' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50335519
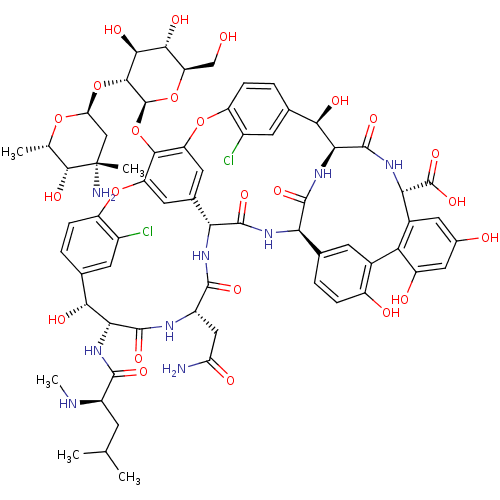
((S)-3,6-Diamino-hexanoic acid {(3S,9S,12S,15S)-3-(...)Show SMILES CN[C@H](CC(C)C)C(=O)N[C@@H]1[C@H](O)c2ccc(Oc3cc4cc(Oc5ccc(cc5Cl)[C@@H](O)[C@@H]5NC(=O)[C@H](NC(=O)[C@@H]4NC(=O)[C@H](CC(N)=O)NC1=O)c1ccc(O)c(c1)-c1c(O)cc(O)cc1[C@H](NC5=O)C(O)=O)c3O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1)c(Cl)c2 |r| Show InChI InChI=1S/C66H75Cl2N9O24/c1-23(2)12-34(71-5)58(88)76-49-51(83)26-7-10-38(32(67)14-26)97-40-16-28-17-41(55(40)101-65-56(54(86)53(85)42(22-78)99-65)100-44-21-66(4,70)57(87)24(3)96-44)98-39-11-8-27(15-33(39)68)52(84)50-63(93)75-48(64(94)95)31-18-29(79)19-37(81)45(31)30-13-25(6-9-36(30)80)46(60(90)77-50)74-61(91)47(28)73-59(89)35(20-43(69)82)72-62(49)92/h6-11,13-19,23-24,34-35,42,44,46-54,56-57,65,71,78-81,83-87H,12,20-22,70H2,1-5H3,(H2,69,82)(H,72,92)(H,73,89)(H,74,91)(H,75,93)(H,76,88)(H,77,90)(H,94,95)/t24-,34+,35-,42+,44-,46+,47+,48-,49+,50-,51+,52+,53+,54-,56+,57+,65-,66-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 41.4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay |
Bioorg Med Chem Lett 14: 773-7 (2004)
BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50484355
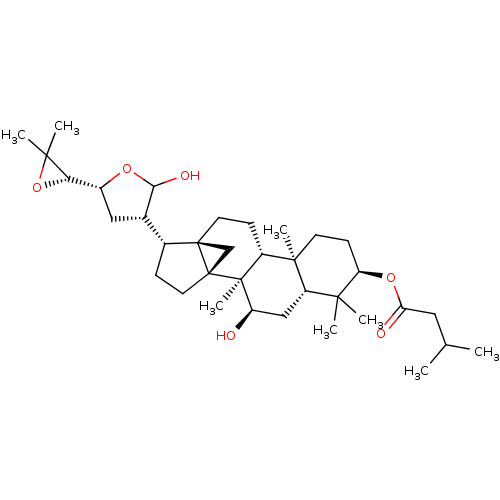
(Skimmiarepin A)Show SMILES [H][C@]1(OC1(C)C)[C@@]1([H])C[C@]([H])(C(O)O1)[C@@]1([H])CC[C@]23C[C@]12CC[C@]1([H])[C@@]2(C)CC[C@@H](OC(=O)CC(C)C)C(C)(C)[C@]2([H])C[C@@H](O)[C@@]31C |r| Show InChI InChI=1S/C35H56O6/c1-19(2)15-27(37)40-26-11-12-32(7)23-10-13-34-18-35(34,33(23,8)25(36)17-24(32)30(26,3)4)14-9-21(34)20-16-22(39-29(20)38)28-31(5,6)41-28/h19-26,28-29,36,38H,9-18H2,1-8H3/t20-,21+,22+,23+,24-,25+,26+,28-,29?,32+,33-,34+,35+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 74: 1894-901 (2011)
Article DOI: 10.1021/np200370z
BindingDB Entry DOI: 10.7270/Q2KW5JVK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50484356
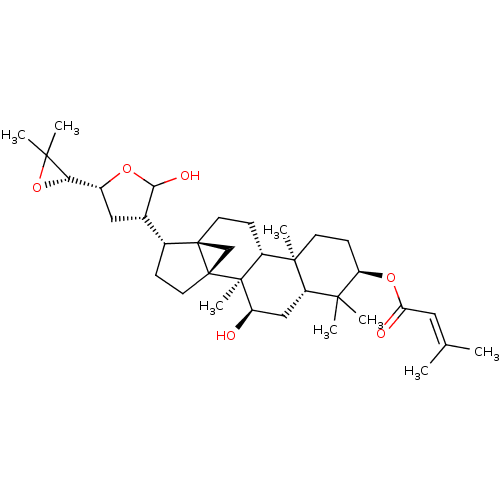
(Skimmiarepin C)Show SMILES [H][C@]1([#8]C1([#6])[#6])[C@@]1([H])[#6][C@]([H])([#6](-[#8])-[#8]1)[C@@]1([H])[#6]-[#6][C@]23[#6][C@]12[#6]-[#6][C@]1([H])[C@@]2([#6])[#6]-[#6]-[#6@@H](-[#8]-[#6](=O)\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@]2([H])[#6]-[#6@@H](-[#8])[C@@]31[#6] |r| Show InChI InChI=1S/C35H54O6/c1-19(2)15-27(37)40-26-11-12-32(7)23-10-13-34-18-35(34,33(23,8)25(36)17-24(32)30(26,3)4)14-9-21(34)20-16-22(39-29(20)38)28-31(5,6)41-28/h15,20-26,28-29,36,38H,9-14,16-18H2,1-8H3/t20-,21+,22+,23+,24-,25+,26+,28-,29?,32+,33-,34+,35+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 74: 1894-901 (2011)
Article DOI: 10.1021/np200370z
BindingDB Entry DOI: 10.7270/Q2KW5JVK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50485671
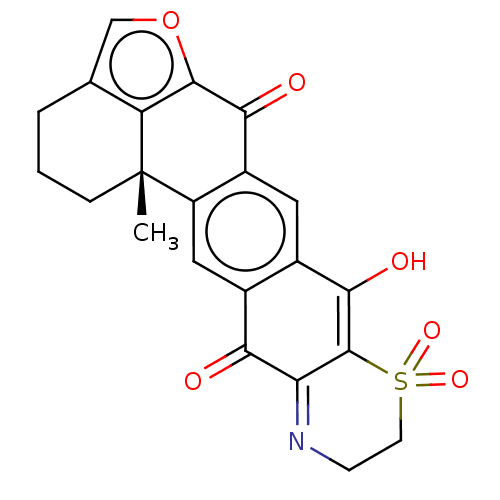
(ADOCIAQUINONE A | Adociaquinones A)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(O)=C4C(=NCCS4(=O)=O)C(=O)c3cc21 |c:18,20| Show InChI InChI=1S/C22H17NO6S/c1-22-4-2-3-10-9-29-20(15(10)22)18(25)13-7-11-12(8-14(13)22)17(24)16-21(19(11)26)30(27,28)6-5-23-16/h7-9,26H,2-6H2,1H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of 1,10-phenanthroline-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50267086

(Adociaquinone B | CHEMBL476648)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)C4=NCCS(=O)(=O)C4C(=O)c3cc21 |r,t:19| Show InChI InChI=1S/C22H17NO6S/c1-22-4-2-3-10-9-29-20(15(10)22)18(25)13-7-11-12(8-14(13)22)19(26)21-16(17(11)24)23-5-6-30(21,27)28/h7-9,21H,2-6H2,1H3/t21?,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of 1,10-phenanthroline-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50485669
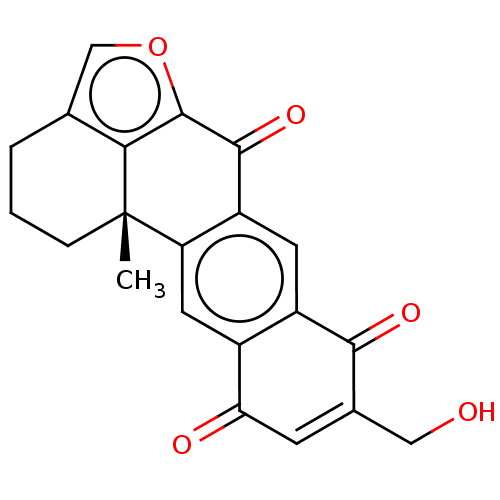
((+)-14-Hydroxymethylxestoquinone | CHEMBL2153503)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)C(CO)=CC(=O)c3cc21 |r,c:21| Show InChI InChI=1S/C21H16O5/c1-21-4-2-3-10-9-26-20(17(10)21)19(25)14-6-13-12(7-15(14)21)16(23)5-11(8-22)18(13)24/h5-7,9,22H,2-4,8H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of 1,10-phenanthroline-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50485670
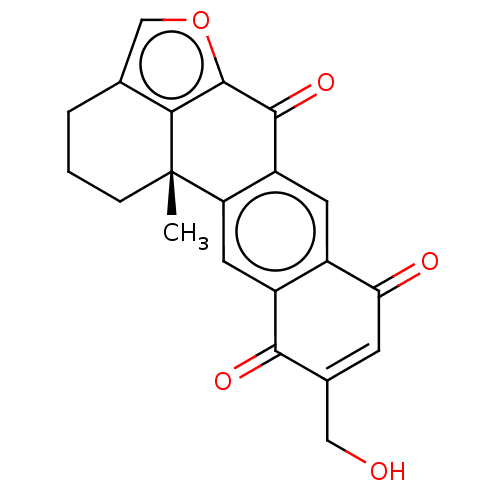
((+)-15-Hydroxymethylxestoquinone | CHEMBL2153504)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)C=C(CO)C(=O)c3cc21 |r,t:19| Show InChI InChI=1S/C21H16O5/c1-21-4-2-3-10-9-26-20(17(10)21)19(25)14-6-12-13(7-15(14)21)18(24)11(8-22)5-16(12)23/h5-7,9,22H,2-4,8H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of 1,10-phenanthroline-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50485669

((+)-14-Hydroxymethylxestoquinone | CHEMBL2153503)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)C(CO)=CC(=O)c3cc21 |r,c:21| Show InChI InChI=1S/C21H16O5/c1-21-4-2-3-10-9-26-20(17(10)21)19(25)14-6-13-12(7-15(14)21)16(23)5-11(8-22)18(13)24/h5-7,9,22H,2-4,8H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50103513
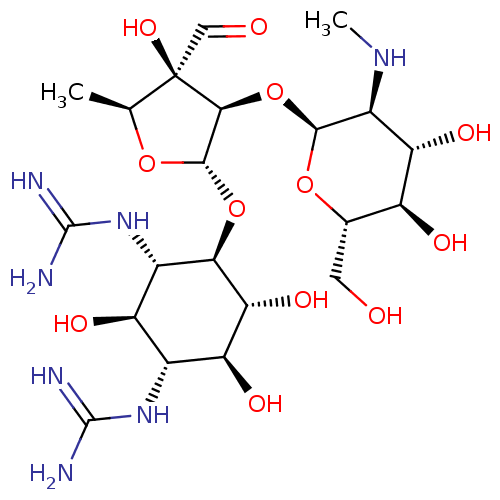
(CHEBI:17076 | Chemform | Gerox | NSC-14083 | Strep...)Show SMILES CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@H]1[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](NC(N)=N)[C@@H](O)[C@@H]2NC(N)=N)O[C@@H](C)[C@]1(O)C=O Show InChI InChI=1S/C21H39N7O12/c1-5-21(36,4-30)16(40-17-9(26-2)13(34)10(31)6(3-29)38-17)18(37-5)39-15-8(28-20(24)25)11(32)7(27-19(22)23)12(33)14(15)35/h4-18,26,29,31-36H,3H2,1-2H3,(H4,22,23,27)(H4,24,25,28)/t5-,6-,7+,8-,9-,10-,11+,12-,13-,14+,15+,16-,17-,18-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay |
Bioorg Med Chem Lett 14: 773-7 (2004)
BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50485670

((+)-15-Hydroxymethylxestoquinone | CHEMBL2153504)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)C=C(CO)C(=O)c3cc21 |r,t:19| Show InChI InChI=1S/C21H16O5/c1-21-4-2-3-10-9-26-20(17(10)21)19(25)14-6-12-13(7-15(14)21)18(24)11(8-22)5-16(12)23/h5-7,9,22H,2-4,8H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50485673
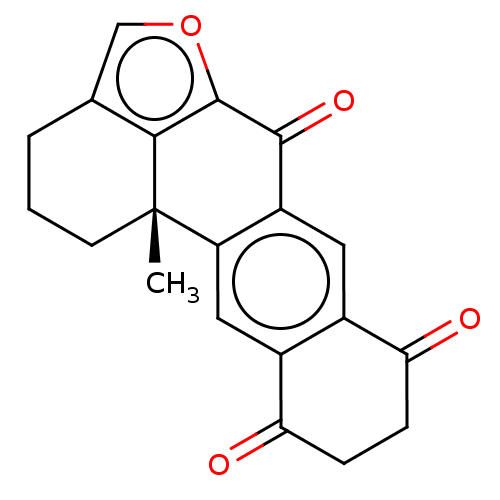
((+)-14,15-Dihydroxestoquinone | CHEMBL2153505)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)CCC(=O)c3cc21 |r| Show InChI InChI=1S/C20H16O4/c1-20-6-2-3-10-9-24-19(17(10)20)18(23)13-7-11-12(8-14(13)20)16(22)5-4-15(11)21/h7-9H,2-6H2,1H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of 1,10-phenanthroline-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50267086

(Adociaquinone B | CHEMBL476648)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)C4=NCCS(=O)(=O)C4C(=O)c3cc21 |r,t:19| Show InChI InChI=1S/C22H17NO6S/c1-22-4-2-3-10-9-29-20(15(10)22)18(25)13-7-11-12(8-14(13)22)19(26)21-16(17(11)24)23-5-6-30(21,27)28/h7-9,21H,2-6H2,1H3/t21?,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50484356

(Skimmiarepin C)Show SMILES [H][C@]1([#8]C1([#6])[#6])[C@@]1([H])[#6][C@]([H])([#6](-[#8])-[#8]1)[C@@]1([H])[#6]-[#6][C@]23[#6][C@]12[#6]-[#6][C@]1([H])[C@@]2([#6])[#6]-[#6]-[#6@@H](-[#8]-[#6](=O)\[#6]=[#6](\[#6])-[#6])C([#6])([#6])[C@]2([H])[#6]-[#6@@H](-[#8])[C@@]31[#6] |r| Show InChI InChI=1S/C35H54O6/c1-19(2)15-27(37)40-26-11-12-32(7)23-10-13-34-18-35(34,33(23,8)25(36)17-24(32)30(26,3)4)14-9-21(34)20-16-22(39-29(20)38)28-31(5,6)41-28/h15,20-26,28-29,36,38H,9-14,16-18H2,1-8H3/t20-,21+,22+,23+,24-,25+,26+,28-,29?,32+,33-,34+,35+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of 1, 10-phenanthroline-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 74: 1894-901 (2011)
Article DOI: 10.1021/np200370z
BindingDB Entry DOI: 10.7270/Q2KW5JVK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50485671

(ADOCIAQUINONE A | Adociaquinones A)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(O)=C4C(=NCCS4(=O)=O)C(=O)c3cc21 |c:18,20| Show InChI InChI=1S/C22H17NO6S/c1-22-4-2-3-10-9-29-20(15(10)22)18(25)13-7-11-12(8-14(13)22)17(24)16-21(19(11)26)30(27,28)6-5-23-16/h7-9,26H,2-6H2,1H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50398330
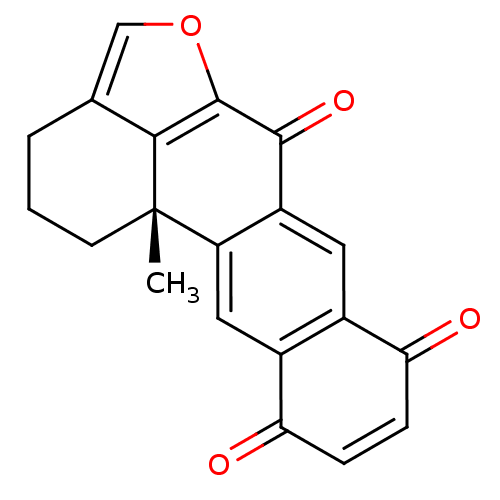
(XESTOQUINONE)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)C=CC(=O)c3cc21 |r,c:19| Show InChI InChI=1S/C20H14O4/c1-20-6-2-3-10-9-24-19(17(10)20)18(23)13-7-11-12(8-14(13)20)16(22)5-4-15(11)21/h4-5,7-9H,2-3,6H2,1H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of 1,10-phenanthroline-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50327206

((2S,5S,8S,11S,14S,17S,20S,23S,26S,29S)-11-((1H-ind...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(O)=O |r| Show InChI InChI=1S/C60H90N12O17/c1-31(2)23-41(66-57(85)45(28-49(77)78)68-58(86)47(30-73)71-55(83)43(26-35-15-9-8-10-16-35)69-59(87)50(34(7)74)72-51(79)38(62)20-21-48(75)76)53(81)67-44(27-36-29-63-39-18-12-11-17-37(36)39)56(84)64-40(19-13-14-22-61)52(80)65-42(24-32(3)4)54(82)70-46(60(88)89)25-33(5)6/h8-12,15-18,29,31-34,38,40-47,50,63,73-74H,13-14,19-28,30,61-62H2,1-7H3,(H,64,84)(H,65,80)(H,66,85)(H,67,81)(H,68,86)(H,69,87)(H,70,82)(H,71,83)(H,72,79)(H,75,76)(H,77,78)(H,88,89)/t34-,38+,40+,41+,42+,43+,44+,45+,46+,47+,50+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p53 binding to MDMX by ELISA |
Bioorg Med Chem 18: 6099-108 (2010)
Article DOI: 10.1016/j.bmc.2010.06.053
BindingDB Entry DOI: 10.7270/Q2GM87J8 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50484355

(Skimmiarepin A)Show SMILES [H][C@]1(OC1(C)C)[C@@]1([H])C[C@]([H])(C(O)O1)[C@@]1([H])CC[C@]23C[C@]12CC[C@]1([H])[C@@]2(C)CC[C@@H](OC(=O)CC(C)C)C(C)(C)[C@]2([H])C[C@@H](O)[C@@]31C |r| Show InChI InChI=1S/C35H56O6/c1-19(2)15-27(37)40-26-11-12-32(7)23-10-13-34-18-35(34,33(23,8)25(36)17-24(32)30(26,3)4)14-9-21(34)20-16-22(39-29(20)38)28-31(5,6)41-28/h19-26,28-29,36,38H,9-18H2,1-8H3/t20-,21+,22+,23+,24-,25+,26+,28-,29?,32+,33-,34+,35+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of 1, 10-phenanthroline-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 74: 1894-901 (2011)
Article DOI: 10.1021/np200370z
BindingDB Entry DOI: 10.7270/Q2KW5JVK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50485673

((+)-14,15-Dihydroxestoquinone | CHEMBL2153505)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)CCC(=O)c3cc21 |r| Show InChI InChI=1S/C20H16O4/c1-20-6-2-3-10-9-24-19(17(10)20)18(23)13-7-11-12(8-14(13)20)16(22)5-4-15(11)21/h7-9H,2-6H2,1H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50398330

(XESTOQUINONE)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3C(=O)C=CC(=O)c3cc21 |r,c:19| Show InChI InChI=1S/C20H14O4/c1-20-6-2-3-10-9-24-19(17(10)20)18(23)13-7-11-12(8-14(13)20)16(22)5-4-15(11)21/h4-5,7-9H,2-3,6H2,1H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50327206

((2S,5S,8S,11S,14S,17S,20S,23S,26S,29S)-11-((1H-ind...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(O)=O |r| Show InChI InChI=1S/C60H90N12O17/c1-31(2)23-41(66-57(85)45(28-49(77)78)68-58(86)47(30-73)71-55(83)43(26-35-15-9-8-10-16-35)69-59(87)50(34(7)74)72-51(79)38(62)20-21-48(75)76)53(81)67-44(27-36-29-63-39-18-12-11-17-37(36)39)56(84)64-40(19-13-14-22-61)52(80)65-42(24-32(3)4)54(82)70-46(60(88)89)25-33(5)6/h8-12,15-18,29,31-34,38,40-47,50,63,73-74H,13-14,19-28,30,61-62H2,1-7H3,(H,64,84)(H,65,80)(H,66,85)(H,67,81)(H,68,86)(H,69,87)(H,70,82)(H,71,83)(H,72,79)(H,75,76)(H,77,78)(H,88,89)/t34-,38+,40+,41+,42+,43+,44+,45+,46+,47+,50+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p53 binding to Hdm2 by ELISA |
Bioorg Med Chem 18: 6099-108 (2010)
Article DOI: 10.1016/j.bmc.2010.06.053
BindingDB Entry DOI: 10.7270/Q2GM87J8 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50221769
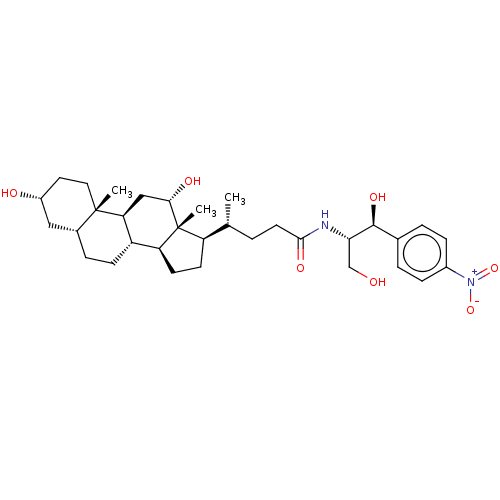
(CHEMBL166184)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(=O)N[C@@H](CO)[C@@H](O)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C33H50N2O7/c1-19(4-13-30(39)34-28(18-36)31(40)20-5-8-22(9-6-20)35(41)42)25-11-12-26-24-10-7-21-16-23(37)14-15-32(21,2)27(24)17-29(38)33(25,26)3/h5-6,8-9,19,21,23-29,31,36-38,40H,4,7,10-18H2,1-3H3,(H,34,39)/t19-,21-,23-,24+,25-,26+,27+,28+,29+,31+,32+,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay |
Bioorg Med Chem Lett 14: 773-7 (2004)
BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50221777
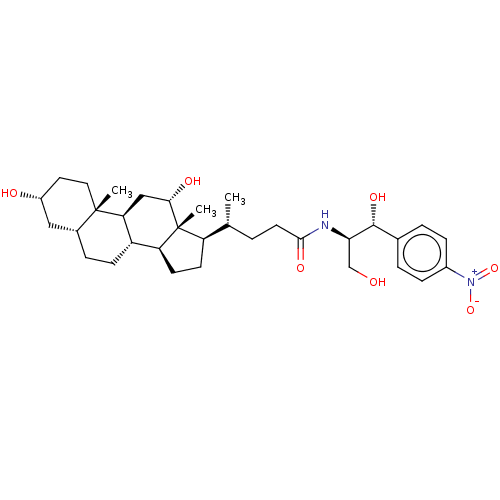
(CHEMBL352932)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(=O)N[C@H](CO)[C@H](O)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C33H50N2O7/c1-19(4-13-30(39)34-28(18-36)31(40)20-5-8-22(9-6-20)35(41)42)25-11-12-26-24-10-7-21-16-23(37)14-15-32(21,2)27(24)17-29(38)33(25,26)3/h5-6,8-9,19,21,23-29,31,36-38,40H,4,7,10-18H2,1-3H3,(H,34,39)/t19-,21-,23-,24+,25-,26+,27+,28-,29+,31-,32+,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay |
Bioorg Med Chem Lett 14: 773-7 (2004)
BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50485672
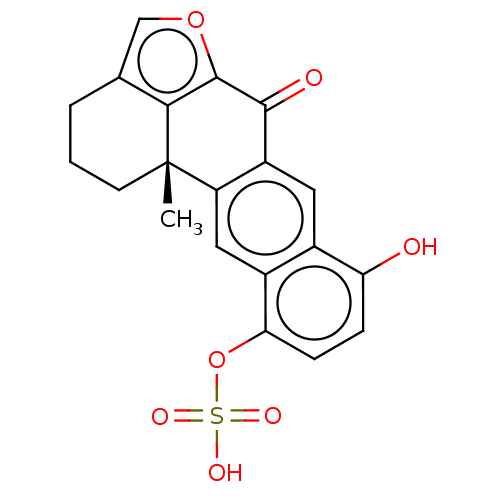
(Xestoquinol Sulfate)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3c(O)ccc(OS(O)(=O)=O)c3cc21 |r| Show InChI InChI=1S/C20H16O7S/c1-20-6-2-3-10-9-26-19(17(10)20)18(22)13-7-11-12(8-14(13)20)16(5-4-15(11)21)27-28(23,24)25/h4-5,7-9,21H,2-3,6H2,1H3,(H,23,24,25)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of 1,10-phenanthroline-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50485672

(Xestoquinol Sulfate)Show SMILES C[C@@]12CCCc3coc(c13)C(=O)c1cc3c(O)ccc(OS(O)(=O)=O)c3cc21 |r| Show InChI InChI=1S/C20H16O7S/c1-20-6-2-3-10-9-26-19(17(10)20)18(22)13-7-11-12(8-14(13)20)16(5-4-15(11)21)27-28(23,24)25/h4-5,7-9,21H,2-3,6H2,1H3,(H,23,24,25)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay |
J Nat Prod 75: 1553-9 (2012)
Article DOI: 10.1021/np3002892
BindingDB Entry DOI: 10.7270/Q2TH8QKK |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50221774
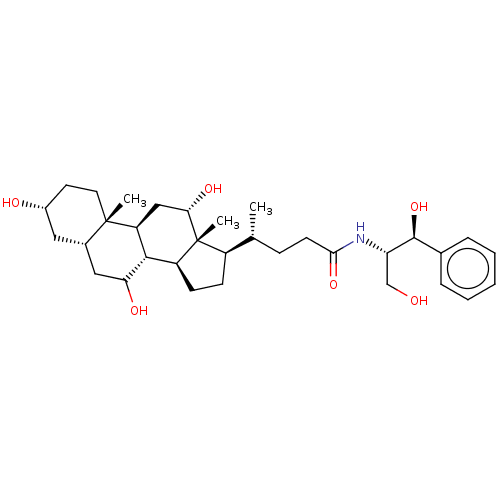
(CHEMBL353406)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])C(O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(=O)N[C@@H](CO)[C@@H](O)c1ccccc1 Show InChI InChI=1S/C33H51NO6/c1-19(9-12-29(39)34-26(18-35)31(40)20-7-5-4-6-8-20)23-10-11-24-30-25(17-28(38)33(23,24)3)32(2)14-13-22(36)15-21(32)16-27(30)37/h4-8,19,21-28,30-31,35-38,40H,9-18H2,1-3H3,(H,34,39)/t19-,21+,22-,23-,24+,25+,26+,27?,28+,30+,31+,32+,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay |
Bioorg Med Chem Lett 14: 773-7 (2004)
BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50221746
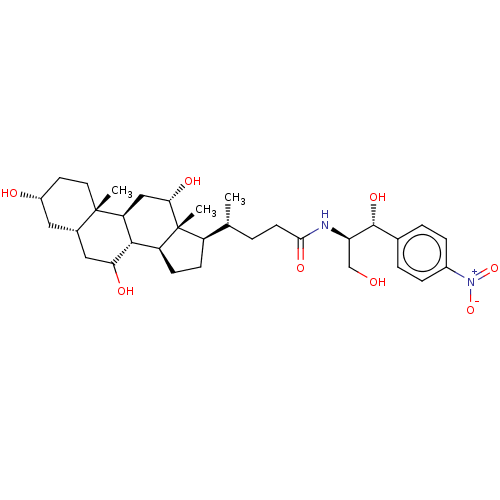
(CHEMBL354064)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])C(O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(=O)N[C@H](CO)[C@H](O)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C33H50N2O8/c1-18(4-11-29(40)34-26(17-36)31(41)19-5-7-21(8-6-19)35(42)43)23-9-10-24-30-25(16-28(39)33(23,24)3)32(2)13-12-22(37)14-20(32)15-27(30)38/h5-8,18,20,22-28,30-31,36-39,41H,4,9-17H2,1-3H3,(H,34,40)/t18-,20+,22-,23-,24+,25+,26-,27?,28+,30+,31-,32+,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay |
Bioorg Med Chem Lett 14: 773-7 (2004)
BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50221744

(CHEMBL349656)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])C(O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(=O)N[C@H](CO)[C@H](O)c1ccccc1 Show InChI InChI=1S/C33H51NO6/c1-19(9-12-29(39)34-26(18-35)31(40)20-7-5-4-6-8-20)23-10-11-24-30-25(17-28(38)33(23,24)3)32(2)14-13-22(36)15-21(32)16-27(30)37/h4-8,19,21-28,30-31,35-38,40H,9-18H2,1-3H3,(H,34,39)/t19-,21+,22-,23-,24+,25+,26-,27?,28+,30+,31-,32+,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay |
Bioorg Med Chem Lett 14: 773-7 (2004)
BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 |
More data for this
Ligand-Target Pair | |
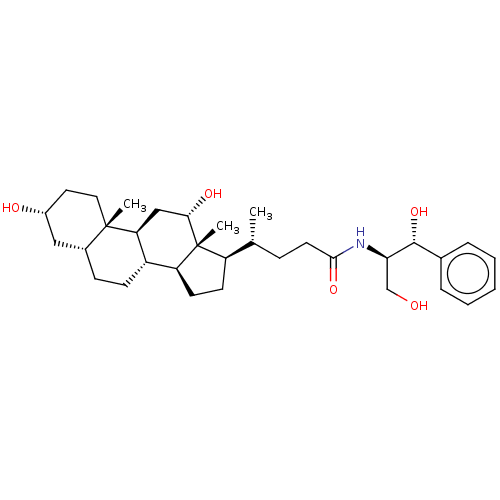
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50221745
(CHEMBL350726)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(=O)N[C@H](CO)[C@H](O)c1ccccc1 Show InChI InChI=1S/C33H51NO5/c1-20(9-14-30(38)34-28(19-35)31(39)21-7-5-4-6-8-21)25-12-13-26-24-11-10-22-17-23(36)15-16-32(22,2)27(24)18-29(37)33(25,26)3/h4-8,20,22-29,31,35-37,39H,9-19H2,1-3H3,(H,34,38)/t20-,22-,23-,24+,25-,26+,27+,28-,29+,31-,32+,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay |
Bioorg Med Chem Lett 14: 773-7 (2004)
BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50327208
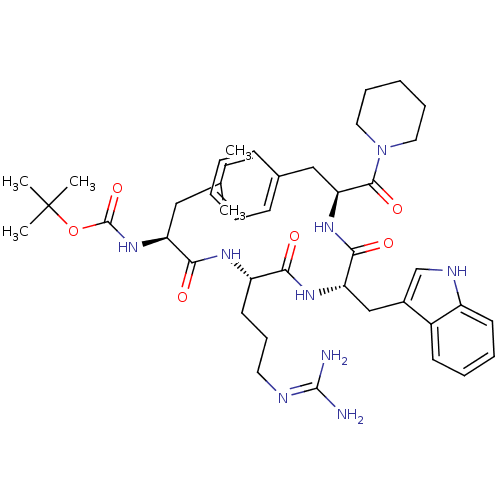
(CHEMBL1256053 | tert-butyl (S)-1-((S)-1-((S)-3-(1H...)Show SMILES CC(C)C[C@H](NC(=O)OC(C)(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCCCC1 |r,wU:4.4,27.26,41.42,wD:16.15,(-4.51,4.31,;-4.51,2.77,;-3.18,2,;-5.84,2,;-5.84,.46,;-7.18,-.31,;-8.51,.46,;-9.84,-.31,;-8.51,2,;-9.84,2.77,;-9.84,4.31,;-10.37,1.33,;-11.36,3.04,;-4.51,-.31,;-4.51,-1.85,;-3.18,.46,;-1.84,-.31,;-1.84,-1.85,;-.51,-2.62,;-.51,-4.16,;.83,-4.93,;.83,-6.47,;-.51,-7.24,;2.16,-7.24,;-.51,.46,;-.51,2,;.83,-.31,;2.16,.46,;2.16,2,;3.49,2.77,;4.9,2.15,;5.93,3.29,;5.16,4.63,;5.64,6.09,;4.61,7.24,;3.1,6.92,;2.62,5.45,;3.65,4.31,;3.89,-.54,;3.89,-2.08,;5.63,.46,;7.36,-.54,;8.69,.23,;8.69,1.77,;7.36,2.54,;7.36,4.08,;8.69,4.85,;10.03,4.08,;10.03,2.54,;7.36,-2.08,;6.03,-2.85,;8.69,-2.85,;10.03,-2.08,;11.36,-2.85,;11.36,-4.39,;10.03,-5.16,;8.69,-4.39,)| Show InChI InChI=1S/C42H61N9O6/c1-27(2)23-33(50-41(56)57-42(3,4)5)37(53)47-32(19-14-20-45-40(43)44)36(52)48-34(25-29-26-46-31-18-11-10-17-30(29)31)38(54)49-35(24-28-15-8-6-9-16-28)39(55)51-21-12-7-13-22-51/h6,8-11,15-18,26-27,32-35,46H,7,12-14,19-25H2,1-5H3,(H,47,53)(H,48,52)(H,49,54)(H,50,56)(H4,43,44,45)/t32-,33-,34-,35-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p53 binding to MDMX by ELISA |
Bioorg Med Chem 18: 6099-108 (2010)
Article DOI: 10.1016/j.bmc.2010.06.053
BindingDB Entry DOI: 10.7270/Q2GM87J8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50327213

((3S,6S,9S,12S,15S,18S)-3-benzyl-6-sec-butyl-15,18-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(C)C |r| Show InChI InChI=1S/C41H52N6O8/c1-6-24(4)35-41(55)45-32(20-26-10-8-7-9-11-26)38(52)43-31(21-27-12-16-29(48)17-13-27)37(51)44-33(22-28-14-18-30(49)19-15-28)39(53)46-34(23(2)3)40(54)42-25(5)36(50)47-35/h7-19,23-25,31-35,48-49H,6,20-22H2,1-5H3,(H,42,54)(H,43,52)(H,44,51)(H,45,55)(H,46,53)(H,47,50)/t24-,25-,31-,32-,33-,34-,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p53 binding to Hdm2 by ELISA |
Bioorg Med Chem 18: 6099-108 (2010)
Article DOI: 10.1016/j.bmc.2010.06.053
BindingDB Entry DOI: 10.7270/Q2GM87J8 |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50327210
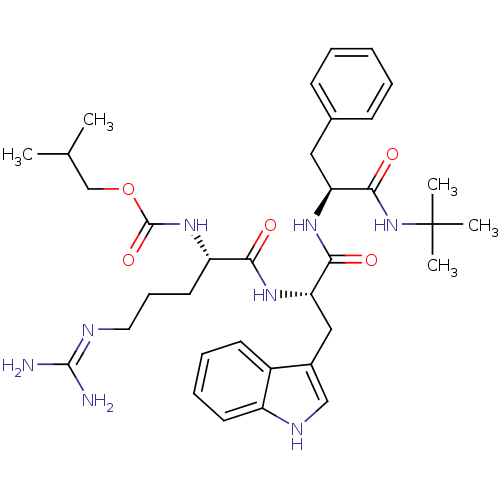
(CHEMBL1256067 | isobutyl (6S,9S,12S)-9-((1H-indol-...)Show SMILES CC(C)COC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC(C)(C)C |r,wU:33.34,8.7,wD:19.18,(25.68,-40.37,;27.03,-41.13,;27.05,-42.69,;28.37,-40.35,;29.72,-41.11,;31.06,-40.32,;31.05,-38.77,;32.45,-41.12,;33.78,-40.33,;33.76,-38.79,;35.08,-38.01,;35.06,-36.46,;36.38,-35.68,;36.36,-34.14,;35.02,-33.39,;37.69,-33.36,;35.11,-41.09,;35.14,-42.62,;36.44,-40.3,;37.79,-41.06,;37.8,-42.59,;39.14,-43.34,;40.55,-42.71,;41.59,-43.84,;40.83,-45.18,;41.33,-46.64,;40.31,-47.79,;38.8,-47.48,;38.3,-46.03,;39.32,-44.88,;39.1,-40.27,;39.09,-38.73,;40.44,-41.02,;41.78,-40.24,;41.75,-38.7,;40.68,-37.58,;41.1,-36.1,;40.04,-35,;38.55,-35.37,;38.13,-36.85,;39.19,-37.96,;43.16,-41.02,;43.18,-42.62,;44.53,-40.21,;45.92,-40.98,;47.28,-40.18,;45.93,-42.54,;47.41,-41.38,)| Show InChI InChI=1S/C35H50N8O5/c1-22(2)21-48-34(47)42-27(16-11-17-38-33(36)37)30(44)41-29(19-24-20-39-26-15-10-9-14-25(24)26)31(45)40-28(32(46)43-35(3,4)5)18-23-12-7-6-8-13-23/h6-10,12-15,20,22,27-29,39H,11,16-19,21H2,1-5H3,(H,40,45)(H,41,44)(H,42,47)(H,43,46)(H4,36,37,38)/t27-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p53 binding to MDMX by ELISA |
Bioorg Med Chem 18: 6099-108 (2010)
Article DOI: 10.1016/j.bmc.2010.06.053
BindingDB Entry DOI: 10.7270/Q2GM87J8 |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50327209
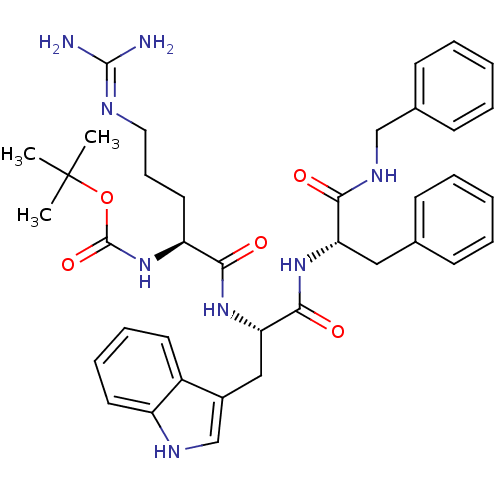
((4S,7S,10S)-7-((1H-indol-3-yl)methyl)-15-amino-4-b...)Show SMILES CC(C)(C)OC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1ccccc1 |r,wU:33.34,8.7,wD:19.18,(-13.47,-29.02,;-12.14,-28.24,;-12.14,-26.68,;-13.48,-27.46,;-10.77,-29,;-9.43,-28.22,;-9.45,-26.66,;-8.04,-29.01,;-6.72,-28.22,;-6.74,-26.69,;-5.42,-25.9,;-5.43,-24.36,;-4.12,-23.57,;-4.13,-22.04,;-5.47,-21.28,;-2.8,-21.24,;-5.38,-28.98,;-5.36,-30.52,;-4.06,-28.19,;-2.71,-28.95,;-2.69,-30.48,;-1.35,-31.23,;.05,-30.6,;1.09,-31.73,;.34,-33.07,;.83,-34.53,;-.19,-35.68,;-1.7,-35.37,;-2.2,-33.92,;-1.17,-32.77,;-1.39,-28.16,;-1.41,-26.62,;-.05,-28.91,;1.28,-28.13,;1.25,-26.59,;.19,-25.47,;.6,-24,;-.46,-22.89,;-1.95,-23.26,;-2.37,-24.74,;-1.31,-25.85,;2.67,-28.91,;2.68,-30.51,;4.03,-28.1,;5.42,-28.88,;6.78,-28.07,;8.15,-28.86,;9.51,-28.06,;9.5,-26.48,;8.12,-25.7,;6.76,-26.5,)| Show InChI InChI=1S/C38H48N8O5/c1-38(2,3)51-37(50)46-30(19-12-20-41-36(39)40)34(48)45-32(22-27-24-42-29-18-11-10-17-28(27)29)35(49)44-31(21-25-13-6-4-7-14-25)33(47)43-23-26-15-8-5-9-16-26/h4-11,13-18,24,30-32,42H,12,19-23H2,1-3H3,(H,43,47)(H,44,49)(H,45,48)(H,46,50)(H4,39,40,41)/t30-,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p53 binding to MDMX by ELISA |
Bioorg Med Chem 18: 6099-108 (2010)
Article DOI: 10.1016/j.bmc.2010.06.053
BindingDB Entry DOI: 10.7270/Q2GM87J8 |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50327211
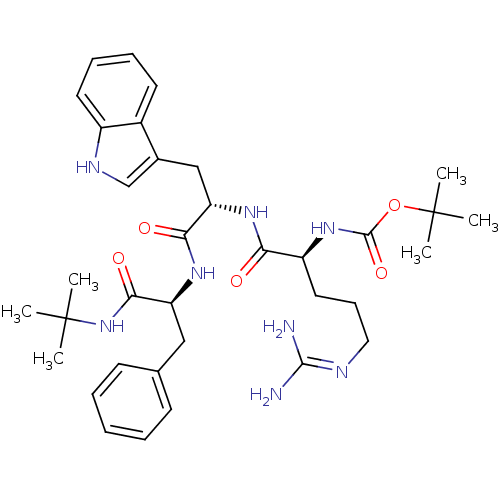
(CHEMBL1256064 | tert-butyl (6S,9S,12S)-9-((1H-indo...)Show SMILES CC(C)(C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)OC(C)(C)C |r,wU:7.15,32.42,wD:18.31,(7.43,-28.91,;6.06,-29.72,;6.08,-31.27,;7.4,-30.48,;4.68,-28.94,;3.31,-29.75,;3.33,-31.35,;1.92,-28.97,;1.89,-27.43,;.83,-26.31,;1.25,-24.84,;.19,-23.73,;-1.31,-24.1,;-1.72,-25.58,;-.66,-26.69,;.59,-29.75,;-.75,-29,;-.77,-27.46,;-2.07,-29.79,;-2.05,-31.32,;-.71,-32.07,;.69,-31.44,;1.74,-32.57,;.98,-33.91,;1.47,-35.37,;.45,-36.52,;-1.06,-36.21,;-1.56,-34.76,;-.53,-33.61,;-3.42,-29.03,;-4.74,-29.82,;-4.72,-31.36,;-6.07,-29.06,;-6.1,-27.53,;-4.77,-26.74,;-4.79,-25.2,;-3.47,-24.41,;-3.49,-22.88,;-4.83,-22.12,;-2.15,-22.08,;-7.4,-29.85,;-8.79,-29.06,;-8.8,-27.5,;-10.13,-29.84,;-11.49,-29.08,;-12.82,-29.86,;-11.5,-27.52,;-12.84,-28.3,)| Show InChI InChI=1S/C35H50N8O5/c1-34(2,3)43-31(46)27(19-22-13-8-7-9-14-22)40-30(45)28(20-23-21-39-25-16-11-10-15-24(23)25)41-29(44)26(17-12-18-38-32(36)37)42-33(47)48-35(4,5)6/h7-11,13-16,21,26-28,39H,12,17-20H2,1-6H3,(H,40,45)(H,41,44)(H,42,47)(H,43,46)(H4,36,37,38)/t26-,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p53 binding to MDMX by ELISA |
Bioorg Med Chem 18: 6099-108 (2010)
Article DOI: 10.1016/j.bmc.2010.06.053
BindingDB Entry DOI: 10.7270/Q2GM87J8 |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50327212
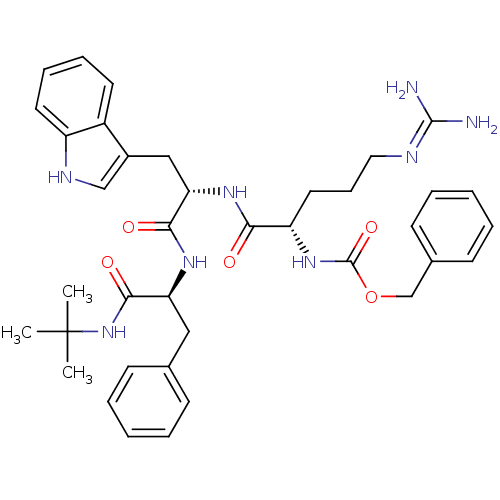
(CHEMBL1256044 | benzyl (6S,9S,12S)-9-((1H-indol-3-...)Show SMILES CC(C)(C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)OCc1ccccc1 |r,wU:7.15,32.42,wD:18.31,(63.42,-49.02,;62.06,-49.83,;62.07,-51.38,;63.39,-50.59,;60.67,-49.05,;59.3,-49.86,;59.32,-51.46,;57.92,-49.08,;57.88,-47.54,;56.82,-46.42,;57.24,-44.95,;56.18,-43.84,;54.69,-44.21,;54.27,-45.69,;55.33,-46.8,;56.58,-49.86,;55.24,-49.11,;55.23,-47.57,;53.93,-49.9,;53.94,-51.43,;55.28,-52.18,;56.68,-51.55,;57.73,-52.68,;56.97,-54.02,;57.47,-55.48,;56.45,-56.63,;54.94,-56.32,;54.44,-54.87,;55.46,-53.72,;52.58,-49.14,;51.25,-49.93,;51.28,-51.47,;49.92,-49.17,;49.89,-47.64,;51.22,-46.85,;51.2,-45.31,;52.52,-44.52,;52.5,-42.99,;51.16,-42.23,;53.83,-42.2,;48.59,-49.96,;47.2,-49.17,;47.19,-47.61,;45.86,-49.95,;44.51,-49.19,;43.17,-49.97,;41.83,-49.2,;40.49,-49.99,;40.5,-51.55,;41.86,-52.31,;43.2,-51.52,)| Show InChI InChI=1S/C38H48N8O5/c1-38(2,3)46-35(49)31(21-25-13-6-4-7-14-25)43-34(48)32(22-27-23-42-29-18-11-10-17-28(27)29)44-33(47)30(19-12-20-41-36(39)40)45-37(50)51-24-26-15-8-5-9-16-26/h4-11,13-18,23,30-32,42H,12,19-22,24H2,1-3H3,(H,43,48)(H,44,47)(H,45,50)(H,46,49)(H4,39,40,41)/t30-,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p53 binding to MDMX by ELISA |
Bioorg Med Chem 18: 6099-108 (2010)
Article DOI: 10.1016/j.bmc.2010.06.053
BindingDB Entry DOI: 10.7270/Q2GM87J8 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50221743
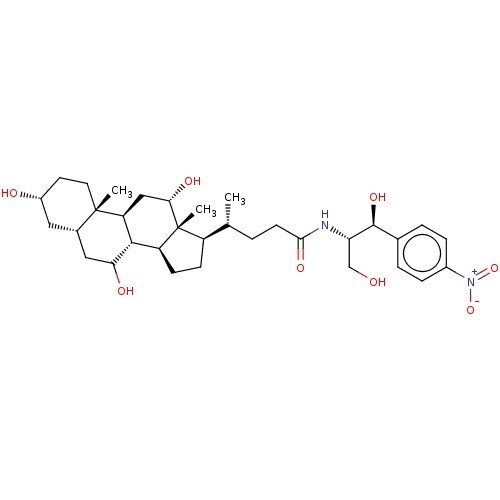
(CHEMBL352152)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])C(O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(=O)N[C@@H](CO)[C@@H](O)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C33H50N2O8/c1-18(4-11-29(40)34-26(17-36)31(41)19-5-7-21(8-6-19)35(42)43)23-9-10-24-30-25(16-28(39)33(23,24)3)32(2)13-12-22(37)14-20(32)15-27(30)38/h5-8,18,20,22-28,30-31,36-39,41H,4,9-17H2,1-3H3,(H,34,40)/t18-,20+,22-,23-,24+,25+,26+,27?,28+,30+,31+,32+,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >4.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay |
Bioorg Med Chem Lett 14: 773-7 (2004)
BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50221775
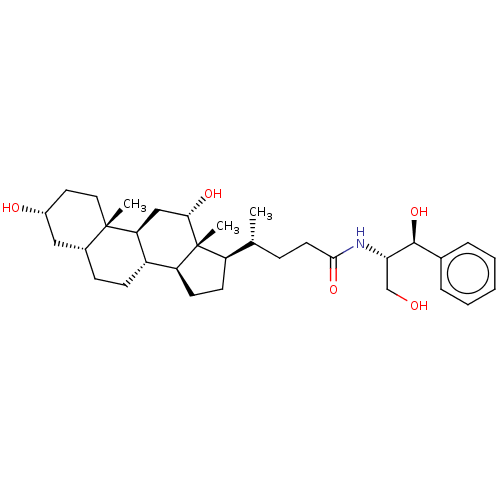
(CHEMBL164621)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(=O)N[C@@H](CO)[C@@H](O)c1ccccc1 Show InChI InChI=1S/C33H51NO5/c1-20(9-14-30(38)34-28(19-35)31(39)21-7-5-4-6-8-21)25-12-13-26-24-11-10-22-17-23(36)15-16-32(22,2)27(24)18-29(37)33(25,26)3/h4-8,20,22-29,31,35-37,39H,9-19H2,1-3H3,(H,34,38)/t20-,22-,23-,24+,25-,26+,27+,28+,29+,31+,32+,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >4.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory
Curated by ChEMBL
| Assay Description
Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay |
Bioorg Med Chem Lett 14: 773-7 (2004)
BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50327207
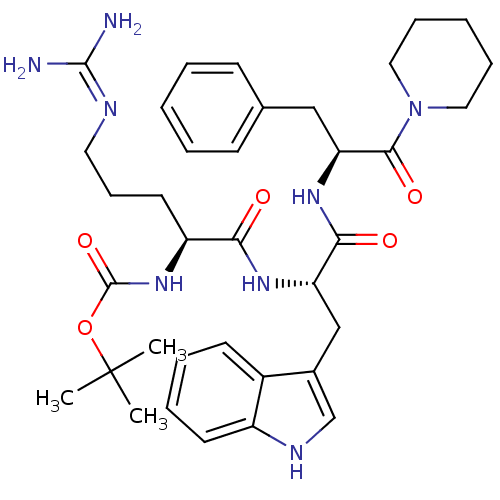
(((S)-5-((S)-3-(1H-indol-3-yl)-1-oxo-1-((S)-1-oxo-3...)Show SMILES CC(C)(C)OC(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCCCC1 |r,wU:33.34,8.7,wD:19.18,(-8.46,-26.11,;-8.38,-27.65,;-9.67,-28.49,;-9.72,-26.87,;-7.01,-28.34,;-5.72,-27.5,;-5.69,-26.03,;-4.34,-28.2,;-3.06,-27.36,;-3.14,-25.82,;-1.85,-24.97,;-1.94,-23.43,;-.65,-22.59,;-.74,-21.05,;-2.12,-20.36,;.55,-20.21,;-1.68,-28.05,;-1.59,-29.58,;-.39,-27.2,;.98,-27.89,;1.07,-29.44,;2.45,-30.13,;3.81,-29.42,;4.9,-30.5,;4.21,-31.88,;4.77,-33.31,;3.81,-34.52,;2.29,-34.28,;1.73,-32.84,;2.69,-31.65,;2.27,-27.05,;2.18,-25.51,;3.65,-27.74,;4.93,-26.9,;4.85,-25.36,;3.68,-24.35,;3.97,-22.85,;2.81,-21.83,;1.35,-22.34,;1.06,-23.85,;2.22,-24.86,;6.31,-27.59,;6.4,-29.13,;7.6,-26.75,;8.96,-27.45,;10.25,-26.62,;10.17,-25.08,;8.8,-24.38,;7.5,-25.22,)| Show InChI InChI=1S/C36H50N8O5/c1-36(2,3)49-35(48)43-28(17-12-18-39-34(37)38)31(45)41-29(22-25-23-40-27-16-9-8-15-26(25)27)32(46)42-30(21-24-13-6-4-7-14-24)33(47)44-19-10-5-11-20-44/h4,6-9,13-16,23,28-30,40H,5,10-12,17-22H2,1-3H3,(H,41,45)(H,42,46)(H,43,48)(H4,37,38,39)/t28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p53 binding to MDMX by ELISA |
Bioorg Med Chem 18: 6099-108 (2010)
Article DOI: 10.1016/j.bmc.2010.06.053
BindingDB Entry DOI: 10.7270/Q2GM87J8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50327206

((2S,5S,8S,11S,14S,17S,20S,23S,26S,29S)-11-((1H-ind...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(O)=O |r| Show InChI InChI=1S/C60H90N12O17/c1-31(2)23-41(66-57(85)45(28-49(77)78)68-58(86)47(30-73)71-55(83)43(26-35-15-9-8-10-16-35)69-59(87)50(34(7)74)72-51(79)38(62)20-21-48(75)76)53(81)67-44(27-36-29-63-39-18-12-11-17-37(36)39)56(84)64-40(19-13-14-22-61)52(80)65-42(24-32(3)4)54(82)70-46(60(88)89)25-33(5)6/h8-12,15-18,29,31-34,38,40-47,50,63,73-74H,13-14,19-28,30,61-62H2,1-7H3,(H,64,84)(H,65,80)(H,66,85)(H,67,81)(H,68,86)(H,69,87)(H,70,82)(H,71,83)(H,72,79)(H,75,76)(H,77,78)(H,88,89)/t34-,38+,40+,41+,42+,43+,44+,45+,46+,47+,50+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to Hdm2 |
Bioorg Med Chem 18: 6099-108 (2010)
Article DOI: 10.1016/j.bmc.2010.06.053
BindingDB Entry DOI: 10.7270/Q2GM87J8 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50327206

((2S,5S,8S,11S,14S,17S,20S,23S,26S,29S)-11-((1H-ind...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(O)=O |r| Show InChI InChI=1S/C60H90N12O17/c1-31(2)23-41(66-57(85)45(28-49(77)78)68-58(86)47(30-73)71-55(83)43(26-35-15-9-8-10-16-35)69-59(87)50(34(7)74)72-51(79)38(62)20-21-48(75)76)53(81)67-44(27-36-29-63-39-18-12-11-17-37(36)39)56(84)64-40(19-13-14-22-61)52(80)65-42(24-32(3)4)54(82)70-46(60(88)89)25-33(5)6/h8-12,15-18,29,31-34,38,40-47,50,63,73-74H,13-14,19-28,30,61-62H2,1-7H3,(H,64,84)(H,65,80)(H,66,85)(H,67,81)(H,68,86)(H,69,87)(H,70,82)(H,71,83)(H,72,79)(H,75,76)(H,77,78)(H,88,89)/t34-,38+,40+,41+,42+,43+,44+,45+,46+,47+,50+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to Hdm2 |
Bioorg Med Chem 18: 6099-108 (2010)
Article DOI: 10.1016/j.bmc.2010.06.053
BindingDB Entry DOI: 10.7270/Q2GM87J8 |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50327206

((2S,5S,8S,11S,14S,17S,20S,23S,26S,29S)-11-((1H-ind...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(O)=O |r| Show InChI InChI=1S/C60H90N12O17/c1-31(2)23-41(66-57(85)45(28-49(77)78)68-58(86)47(30-73)71-55(83)43(26-35-15-9-8-10-16-35)69-59(87)50(34(7)74)72-51(79)38(62)20-21-48(75)76)53(81)67-44(27-36-29-63-39-18-12-11-17-37(36)39)56(84)64-40(19-13-14-22-61)52(80)65-42(24-32(3)4)54(82)70-46(60(88)89)25-33(5)6/h8-12,15-18,29,31-34,38,40-47,50,63,73-74H,13-14,19-28,30,61-62H2,1-7H3,(H,64,84)(H,65,80)(H,66,85)(H,67,81)(H,68,86)(H,69,87)(H,70,82)(H,71,83)(H,72,79)(H,75,76)(H,77,78)(H,88,89)/t34-,38+,40+,41+,42+,43+,44+,45+,46+,47+,50+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity to MDMX |
Bioorg Med Chem 18: 6099-108 (2010)
Article DOI: 10.1016/j.bmc.2010.06.053
BindingDB Entry DOI: 10.7270/Q2GM87J8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data