

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxypeptidase G2 (Pseudomonas aeruginosa) | BDBM50171504 ((S)-2-{4-[Bis-(2-chloro-ethyl)-amino]-2,3,5,6-tetr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against carboxypeptidase G2 from pseudomonas RS16 | J Med Chem 48: 5321-8 (2005) Article DOI: 10.1021/jm0502182 BindingDB Entry DOI: 10.7270/Q2DJ5F5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase G2 (Pseudomonas aeruginosa) | BDBM50171496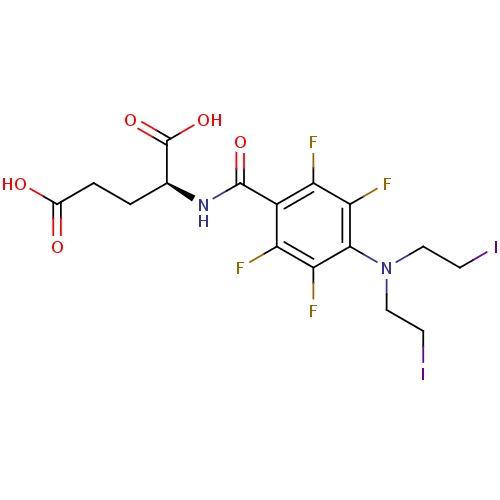 ((S)-2-{4-[Bis-(2-iodo-ethyl)-amino]-2,3,5,6-tetraf...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against carboxypeptidase G2 from pseudomonas RS16 | J Med Chem 48: 5321-8 (2005) Article DOI: 10.1021/jm0502182 BindingDB Entry DOI: 10.7270/Q2DJ5F5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180334 (2-(3,4-Methylenedioxyphenylamino)-6-(3-acetamidoph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25616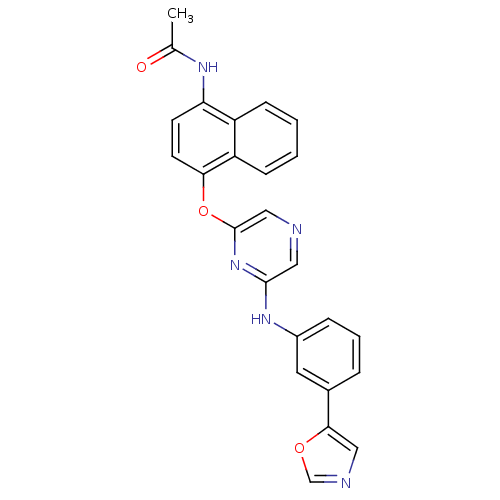 (2,6-Disubstituted Pyrazine, 79 | N-{4-[(6-{[3-(1,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25612 (2,6-Disubstituted Pyrazine, 70 | N-[5-({6-[(3,4,5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180359 (2-(3-Oxo-1,3-dihydroisobenzofuran-5-ylamino)-6-(3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25613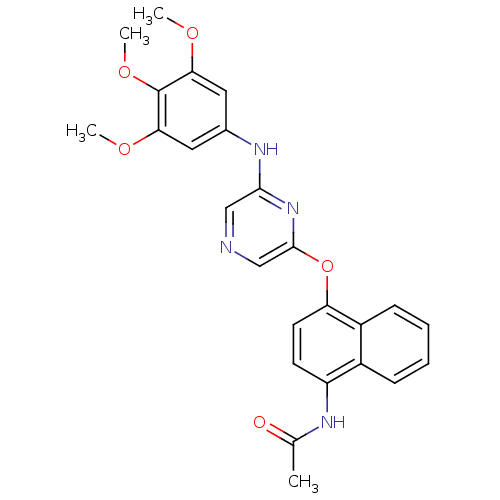 (2,6-Disubstituted Pyrazine, 76 | N-[4-({6-[(3,4,5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25614 (2,6-Disubstituted Pyrazine, 77 | N-{5-[(6-{[3-(1,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180359 (2-(3-Oxo-1,3-dihydroisobenzofuran-5-ylamino)-6-(3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180350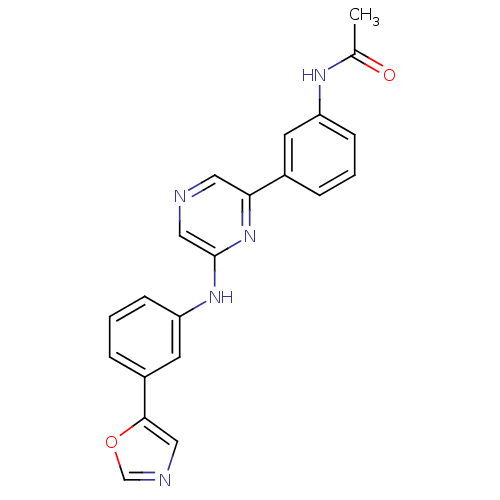 (2-[3-(Oxazol-5-yl)phenylamino]-6-(3-acetamidopheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180353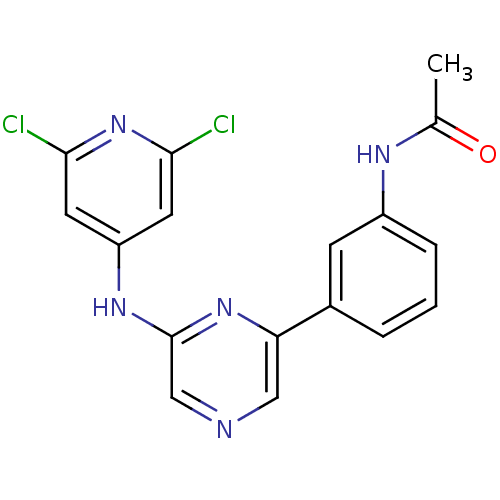 (2-(2,6-Dichloropyridin-4-ylamino)-6-(3-acetamidoph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25570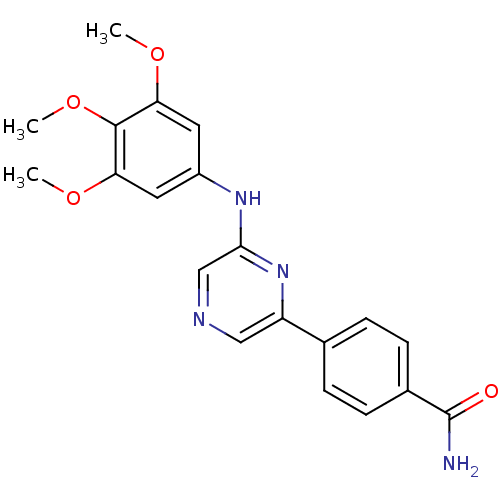 (2,6-Disubstituted Pyrazine, 13 | 4-{6-[(3,4,5-trim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25569 (1-phenyl-3-(4-{6-[(3,4,5-trimethoxyphenyl)amino]py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25580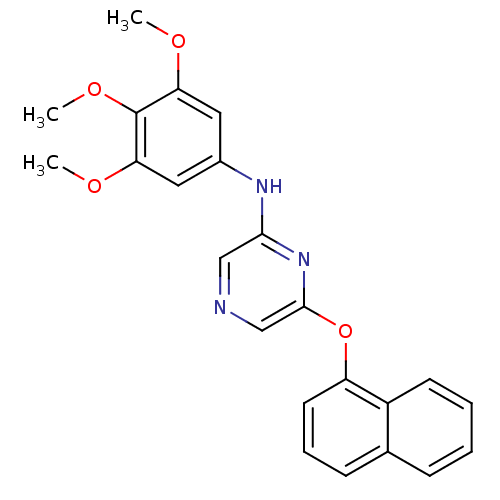 (2,6-Disubstituted Pyrazine, 26 | 6-(naphthalen-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180339 (2-(3,4-Dimethoxyphenylamino)-6-(3-acetamidophenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25609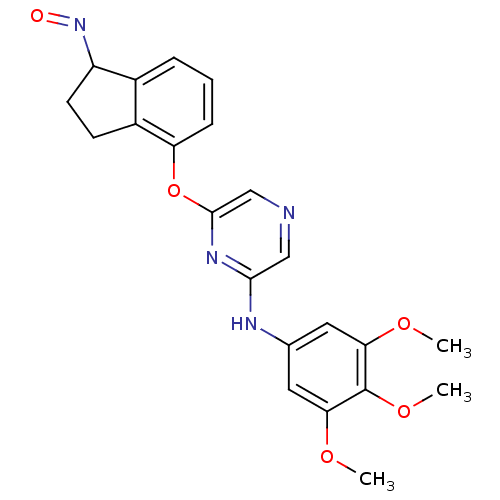 (2,6-Disubstituted Pyrazine, 73 | 6-{[(1E)-1-(hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180330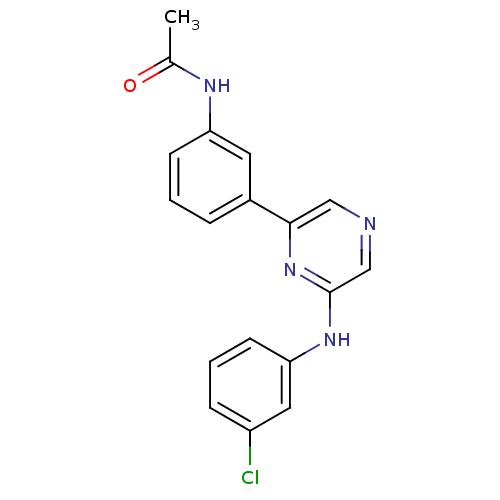 (2-(3-Chlorophenylamino)-6-(3-acetamidophenyl)pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25590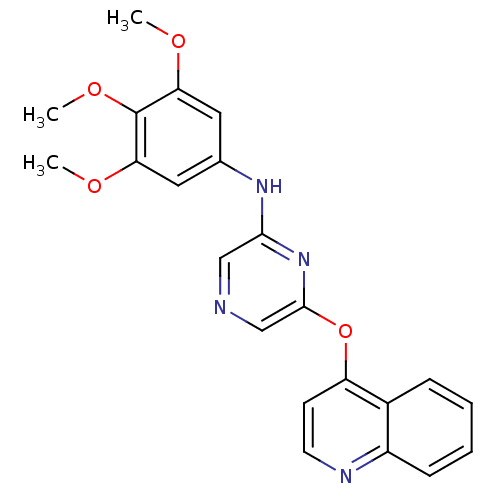 (2,6-Disubstituted Pyrazine, 53 | 6-(quinolin-4-ylo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180354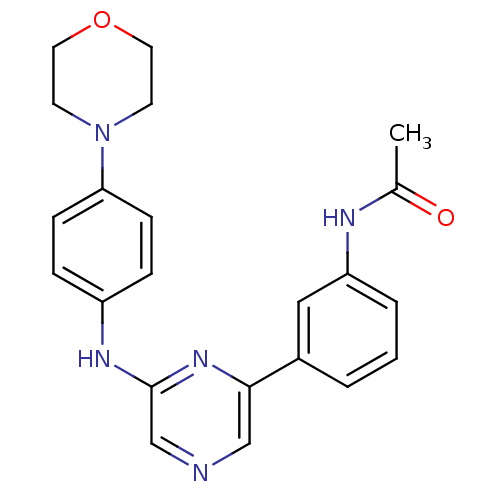 (2-(4-Morpholinophenylamino)-6-(3-acetamidophenyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25615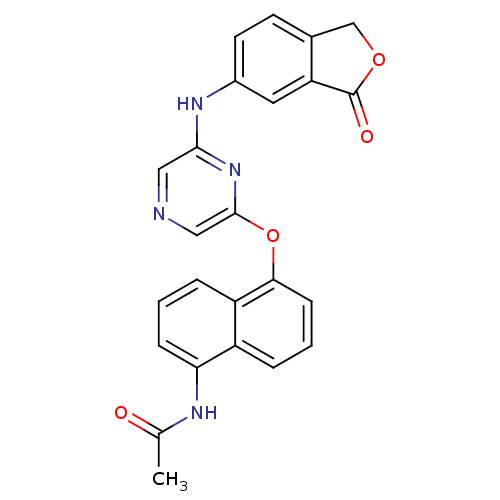 (2,6-Disubstituted Pyrazine, 78 | N-[5-({6-[(3-oxo-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180331 (2-(3,5-Dimethoxyphenylamino)-6-(3-acetamidophenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25562 (2,6-Disubstituted Pyrazine, 4 | 6-(pyridin-4-yl)-N...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180331 (2-(3,5-Dimethoxyphenylamino)-6-(3-acetamidophenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25568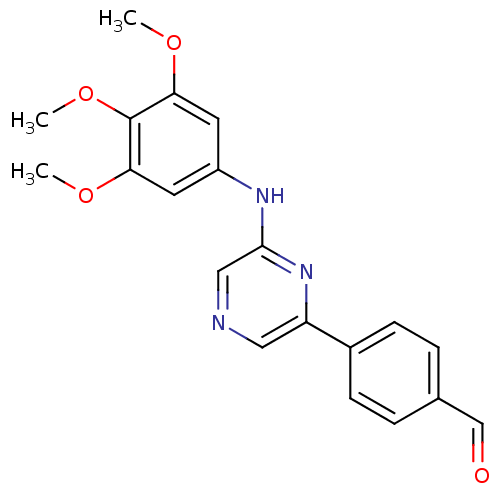 (2,6-Disubstituted Pyrazine, 10 | 4-{6-[(3,4,5-trim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180337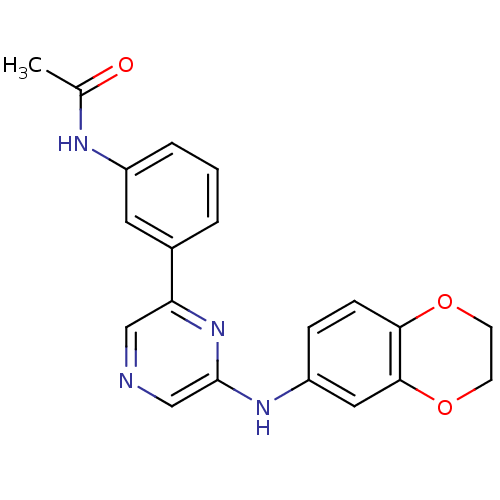 (2-(2,3-Dihydrobenzo[b][1,4]dioxin-6-ylamino)-6-(3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180356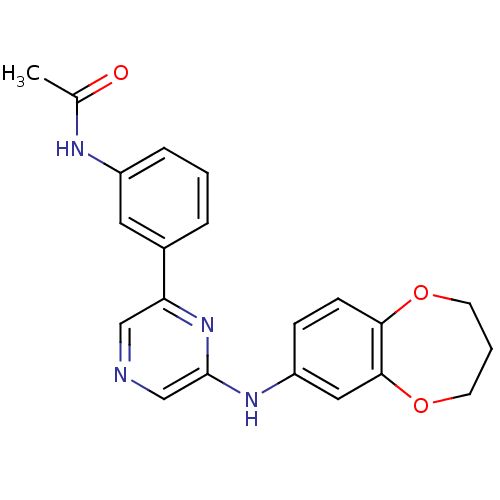 (2-(3,4-Dihydro-2H-benzo[b][1,4]dioxepin-7-ylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25561 (2,6-Disubstituted Pyrazine, 1 | CHEMBL200114 | N-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM25561 (2,6-Disubstituted Pyrazine, 1 | CHEMBL200114 | N-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM25561 (2,6-Disubstituted Pyrazine, 1 | CHEMBL200114 | N-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180358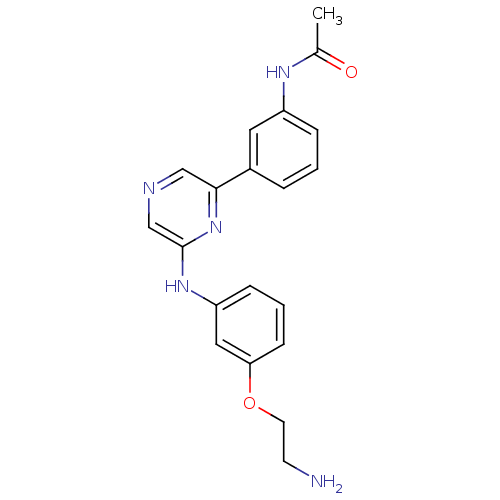 (2-[3-(2-Aminoethoxy)phenylamino]-6-(3-acetamidophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25593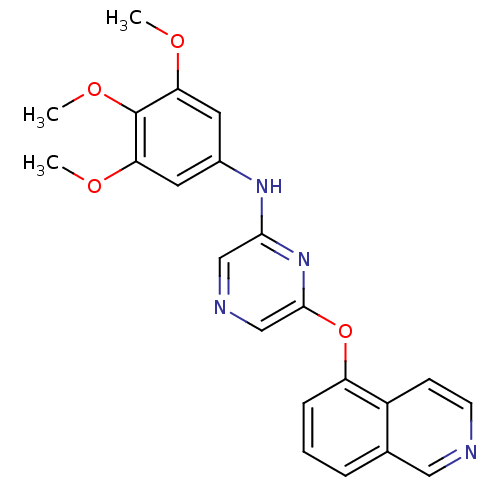 (2,6-Disubstituted Pyrazine, 56 | 6-(isoquinolin-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180332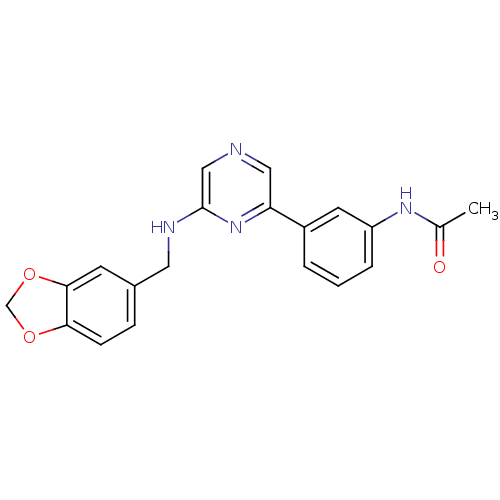 (2-(3,4-Methylenedioxyphenylaminomethyl)-6-(3-aceta...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25564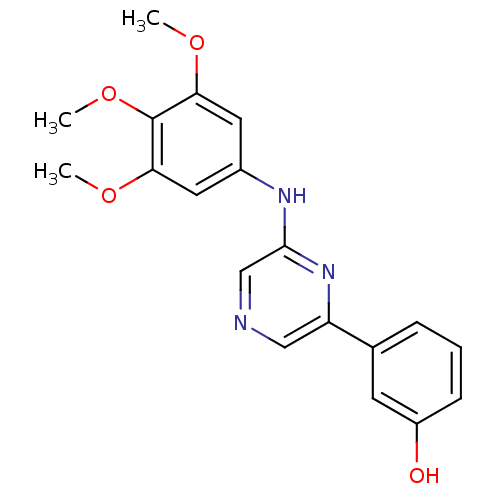 (2,6-Disubstituted Pyrazine, 6 | 3-{6-[(3,4,5-trime...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180341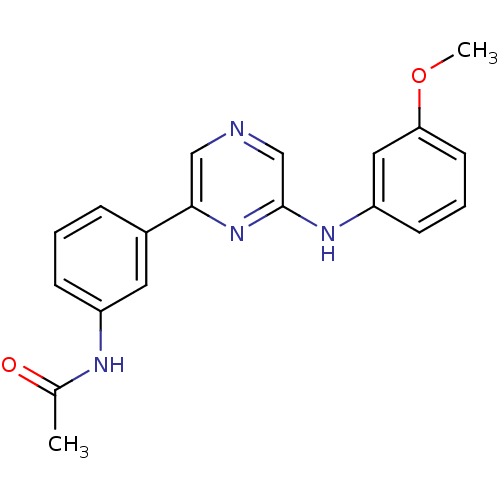 (2-(3-Methoxyphenylamino)-6-(3-acetamidophenyl)pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50367702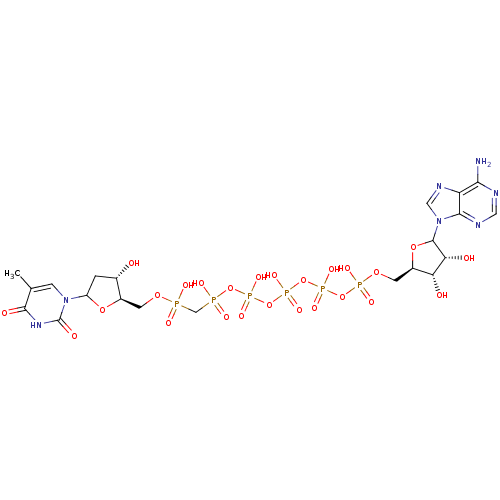 (CHEMBL606084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration required to reduce Thymidylate kinase rate by 50% in human blast cells | J Med Chem 31: 1305-8 (1988) BindingDB Entry DOI: 10.7270/Q2FT8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180333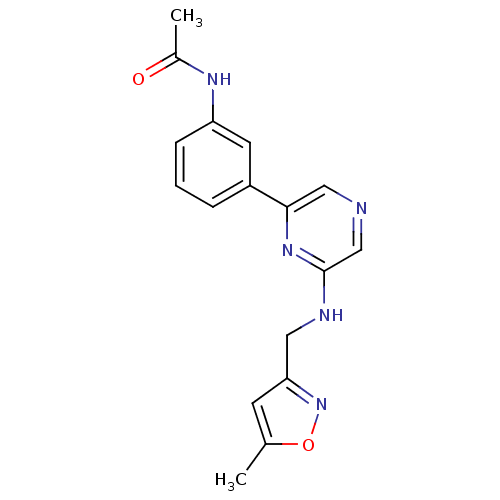 (2-[(5-Methyl-3-isoxazolyl)methylamino]-6-(3-acetam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180356 (2-(3,4-Dihydro-2H-benzo[b][1,4]dioxepin-7-ylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25575 (2,6-Disubstituted Pyrazine, 21 | 2-N-(pyridin-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180341 (2-(3-Methoxyphenylamino)-6-(3-acetamidophenyl)pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25577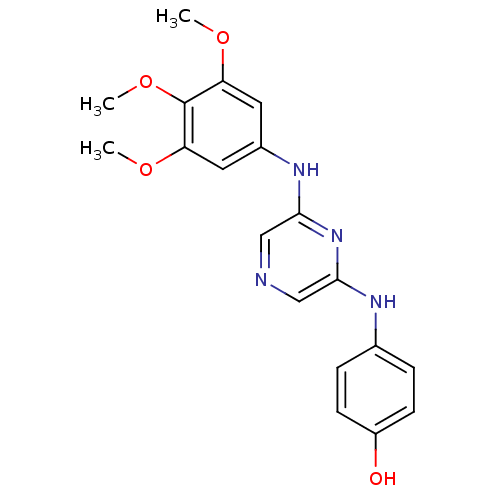 (2,6-Disubstituted Pyrazine, 23 | 4-({6-[(3,4,5-tri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180358 (2-[3-(2-Aminoethoxy)phenylamino]-6-(3-acetamidophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180355 (2-[4-(Trifluoromethoxy)phenylamino]-6-(3-acetamido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180337 (2-(2,3-Dihydrobenzo[b][1,4]dioxin-6-ylamino)-6-(3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against human B-RAF | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM25604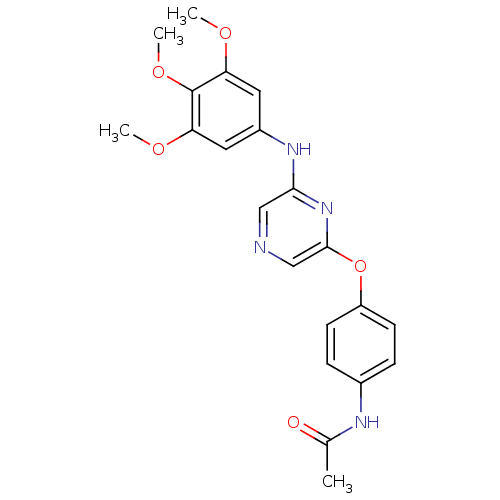 (2,6-Disubstituted Pyrazine, 67 | N-[4-({6-[(3,4,5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research UK Centre for Cancer Therapeutics | Assay Description BRAF kinase activity was quantified using a DELFIA-based MEK1 phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curves... | J Med Chem 51: 3261-74 (2008) Article DOI: 10.1021/jm070776b BindingDB Entry DOI: 10.7270/Q2X34VRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50367696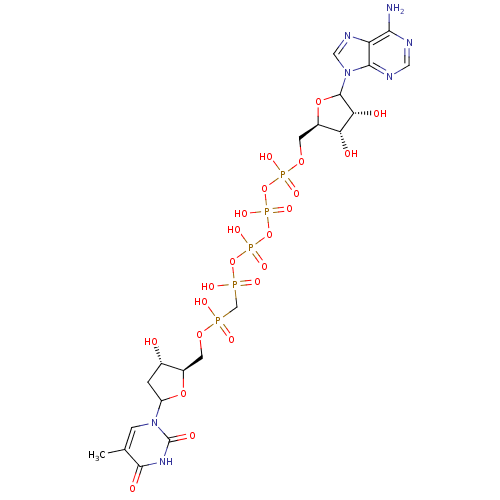 (CHEMBL605435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration required to reduce Thymidylate kinase rate by 50% in human blast cells | J Med Chem 31: 1305-8 (1988) BindingDB Entry DOI: 10.7270/Q2FT8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50367698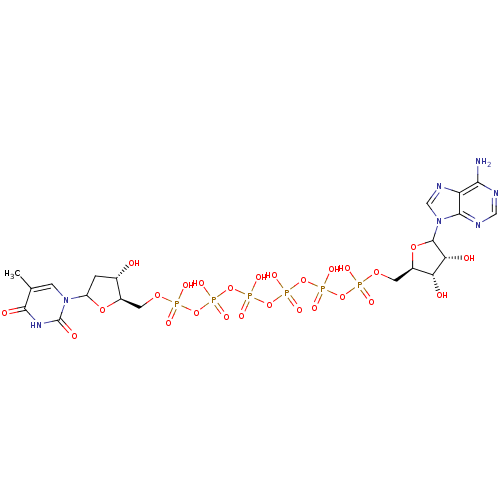 (CHEMBL604405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration required to reduce Thymidylate kinase rate by 50% in human blast cells | J Med Chem 31: 1305-8 (1988) BindingDB Entry DOI: 10.7270/Q2FT8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50180339 (2-(3,4-Dimethoxyphenylamino)-6-(3-acetamidophenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB | J Med Chem 49: 407-16 (2006) Article DOI: 10.1021/jm050983g BindingDB Entry DOI: 10.7270/Q2KD1XH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 136 total ) | Next | Last >> |