

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50239422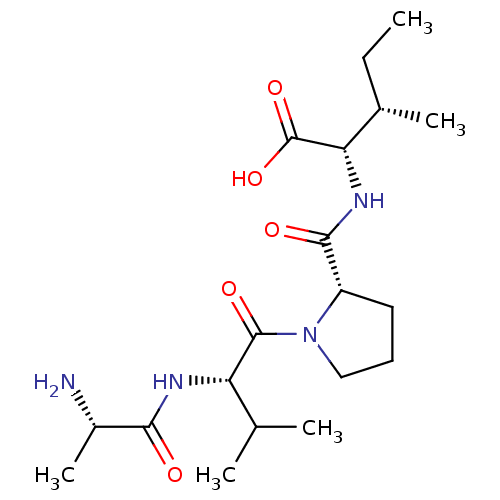 (CHEMBL234346) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of SMAC-derived peptide abuRPFK (5 and 6FAM)-amide interaction with XIAP BIR3 domain (unknown origin) by fluorescence polarization assay | J Med Chem 60: 4611-4625 (2017) Article DOI: 10.1021/acs.jmedchem.6b01877 BindingDB Entry DOI: 10.7270/Q2KK9DX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450049 (CHEMBL4166057) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239500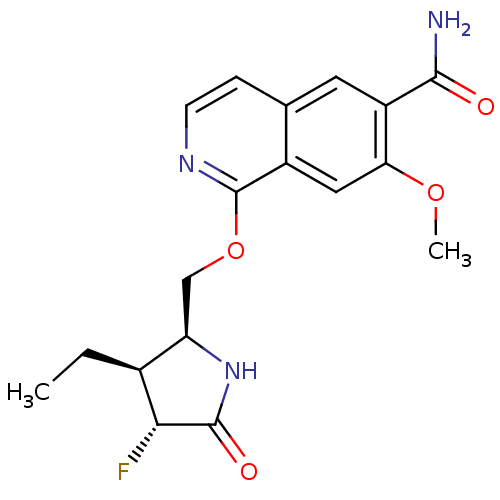 (CHEMBL4066705 | US10329302, Example 337 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450044 (CHEMBL4167141) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450038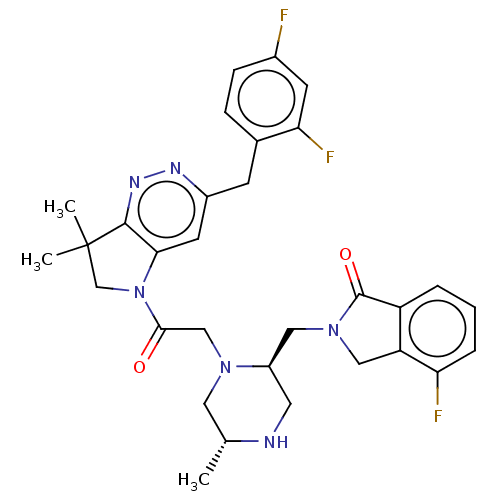 (CHEMBL4171490) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450047 (CHEMBL4169478) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450043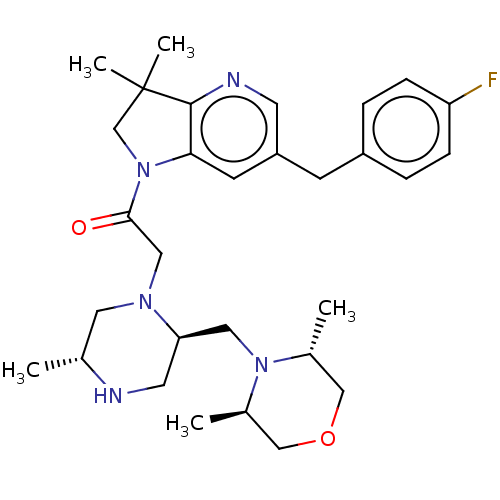 (CHEMBL4166607) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450037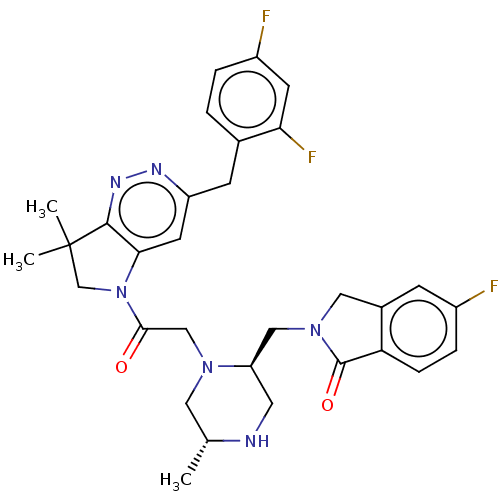 (CHEMBL4164271) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239499 (CHEMBL4081711 | US10329302, Example 344 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50385150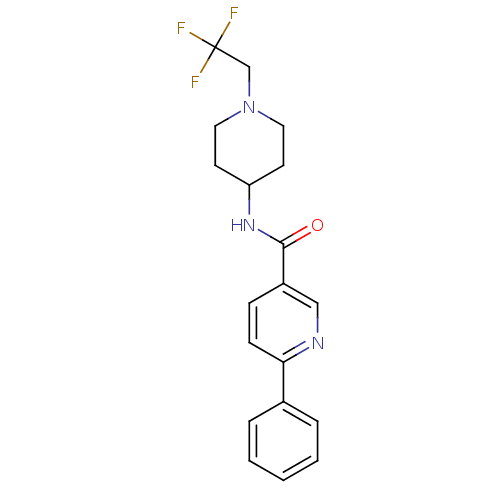 (CHEMBL2035650) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HPGDS | Bioorg Med Chem Lett 22: 3795-9 (2012) Article DOI: 10.1016/j.bmcl.2012.04.004 BindingDB Entry DOI: 10.7270/Q28C9X82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450046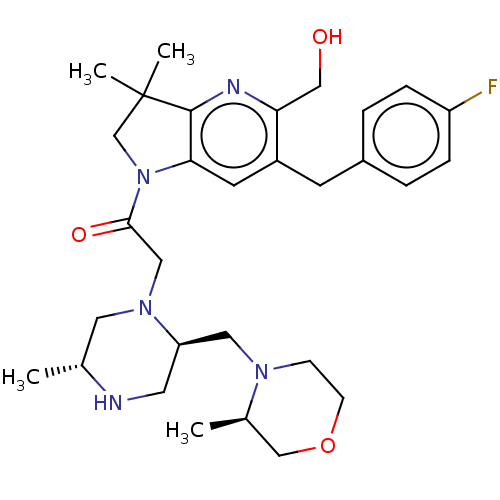 (CHEMBL4173974) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239498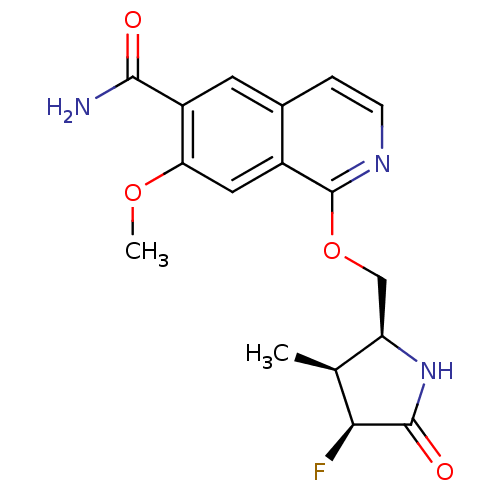 (CHEMBL4093120 | US10329302, Example 189 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50239422 (CHEMBL234346) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of SMAC-derived peptide abuRPFK (5 and 6FAM)-amide interaction with cIAP1 BIR3 domain (unknown origin) by fluorescence polarization assay | J Med Chem 60: 4611-4625 (2017) Article DOI: 10.1021/acs.jmedchem.6b01877 BindingDB Entry DOI: 10.7270/Q2KK9DX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50239425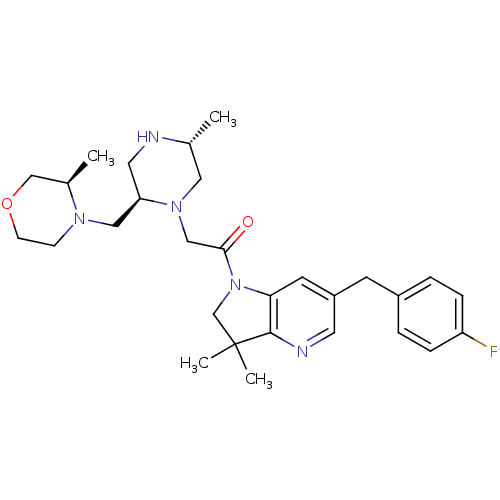 (CHEMBL4064619) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450039 (CHEMBL4160872) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50441356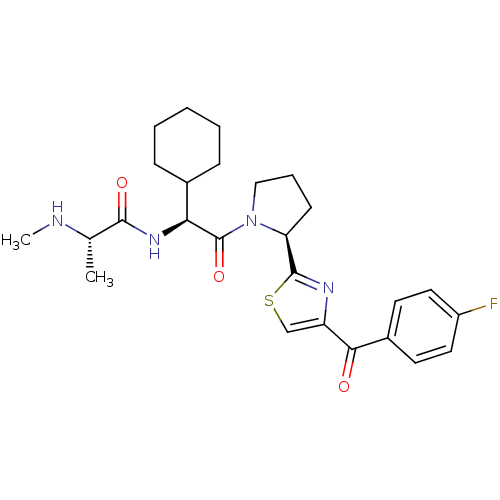 (CHEMBL2431768) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239507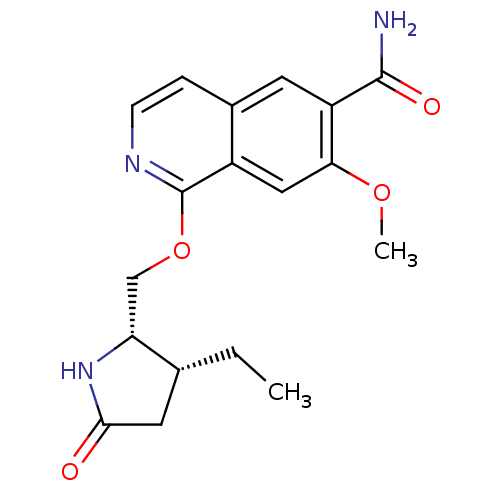 (CHEMBL4091434 | US10329302, Example 246 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450054 (CHEMBL4159232) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239508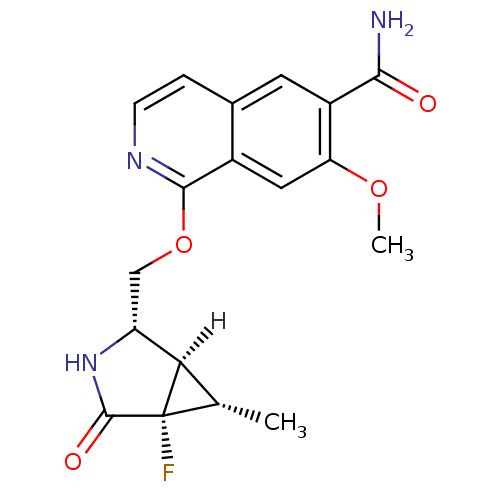 (CHEMBL4085199 | US10329302, Example 309 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450042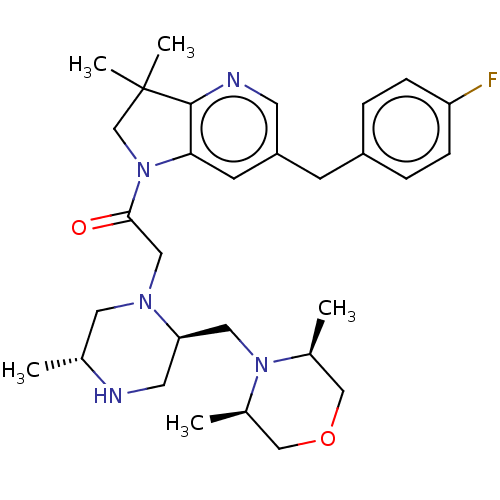 (CHEMBL4168197) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450048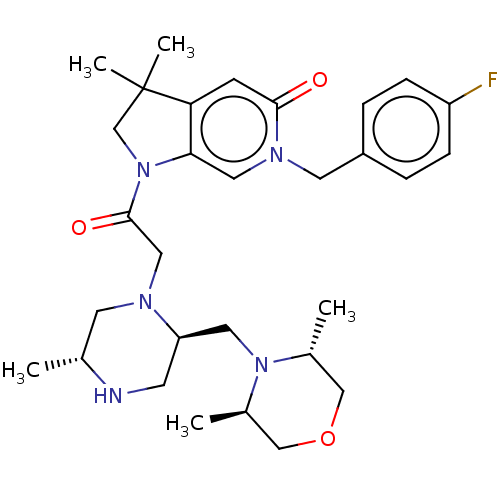 (CHEMBL4177336) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450051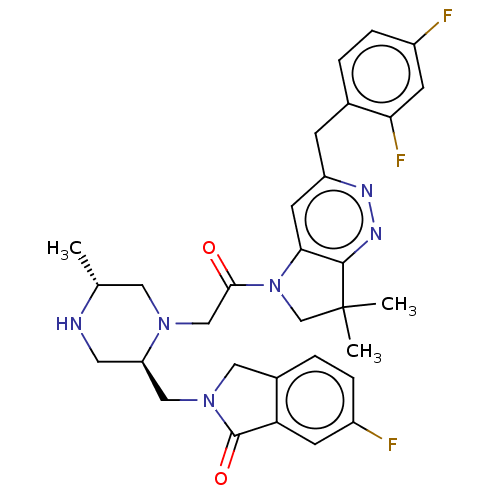 (CHEMBL4174922) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50450047 (CHEMBL4169478) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length FLAG-tagged XIAP (unknown origin) interaction with full length untagged caspase-9 expressed in HEK293 cells after 2 hrs by ... | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450050 (CHEMBL4167717) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418177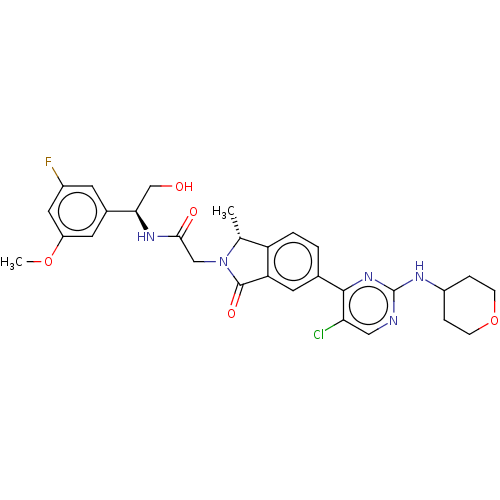 (2-[(1R)-5-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50573875 (CHEMBL4869086) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418177 (2-[(1R)-5-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US11001575 (2021) BindingDB Entry DOI: 10.7270/Q2TB1B01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418177 (2-[(1R)-5-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US10457669 (2019) BindingDB Entry DOI: 10.7270/Q2B27XNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418281 ((2R)-2-[6-(5-chloro-2-{[(2S)-1- hydroxypropan-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US10457669 (2019) BindingDB Entry DOI: 10.7270/Q2B27XNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418281 ((2R)-2-[6-(5-chloro-2-{[(2S)-1- hydroxypropan-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US11001575 (2021) BindingDB Entry DOI: 10.7270/Q2TB1B01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM417954 (US10457669, Example 615) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US10457669 (2019) BindingDB Entry DOI: 10.7270/Q2B27XNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM417999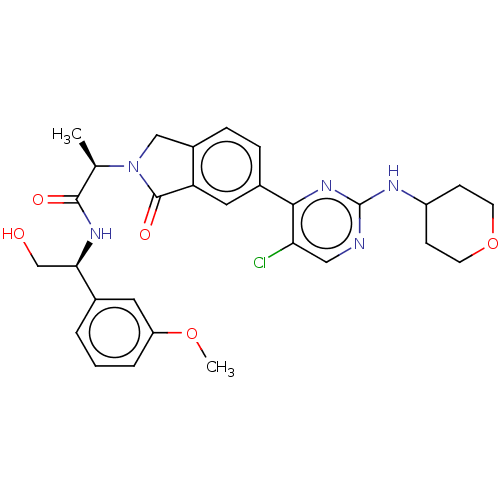 (US10457669, Example 675 | US11001575, Example 675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US10457669 (2019) BindingDB Entry DOI: 10.7270/Q2B27XNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50450053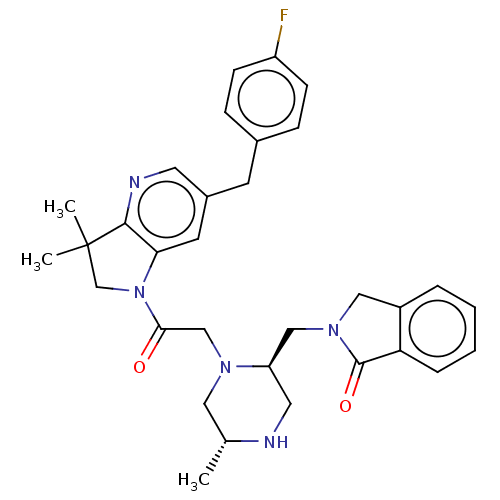 (CHEMBL4175992) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 61: 7314-7329 (2018) Article DOI: 10.1021/acs.jmedchem.8b00900 BindingDB Entry DOI: 10.7270/Q2TT4THH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239491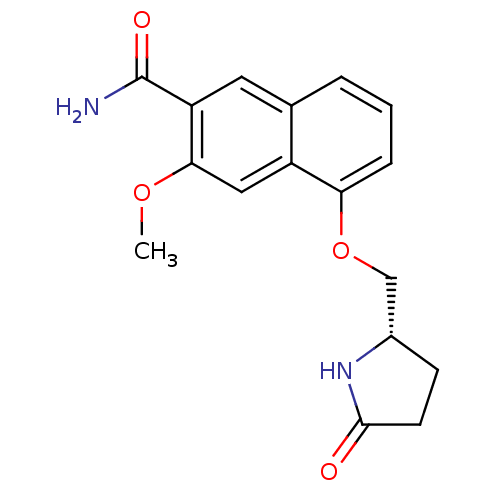 (CHEMBL4083655 | US10329302, Example 173 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239493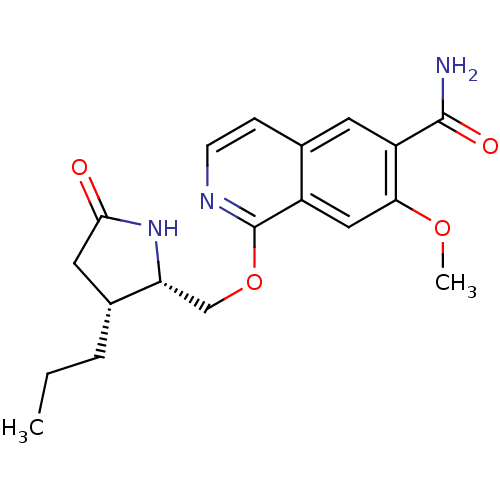 (CHEMBL4103497 | US10329302, Example 312 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM417999 (US10457669, Example 675 | US11001575, Example 675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM417999 (US10457669, Example 675 | US11001575, Example 675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US11001575 (2021) BindingDB Entry DOI: 10.7270/Q2TB1B01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM417953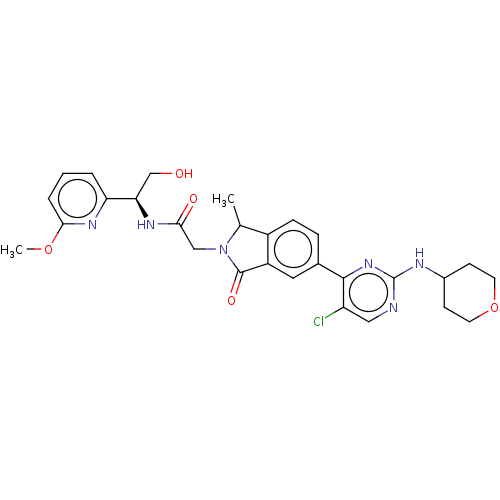 (US10457669, Example 614 | US11001575, Example 616) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US11001575 (2021) BindingDB Entry DOI: 10.7270/Q2TB1B01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418188 (2-[(1R)-5-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US11001575 (2021) BindingDB Entry DOI: 10.7270/Q2TB1B01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418188 (2-[(1R)-5-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US10457669 (2019) BindingDB Entry DOI: 10.7270/Q2B27XNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418230 ((2R)-2-(6-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US11001575 (2021) BindingDB Entry DOI: 10.7270/Q2TB1B01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418223 ((2R)-2-(3-{5-chloro-2-[(2-methyl-2H- 1,2,3-triazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US11001575 (2021) BindingDB Entry DOI: 10.7270/Q2TB1B01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418223 ((2R)-2-(3-{5-chloro-2-[(2-methyl-2H- 1,2,3-triazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US10457669 (2019) BindingDB Entry DOI: 10.7270/Q2B27XNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418230 ((2R)-2-(6-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US10457669 (2019) BindingDB Entry DOI: 10.7270/Q2B27XNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418305 ((2R)-2-(6-{5-chloro-2-[(2- methylpyrimidin-4-yl)am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US10457669 (2019) BindingDB Entry DOI: 10.7270/Q2B27XNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418307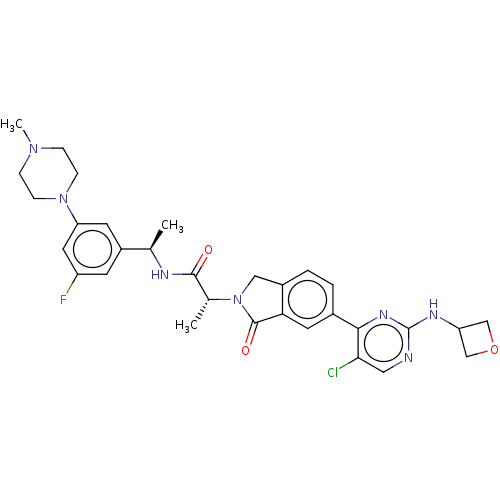 ((2R)-2-(6-}5-chloro-2-[(oxetan-3- yl)amino]pyrimid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US10457669 (2019) BindingDB Entry DOI: 10.7270/Q2B27XNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418229 ((2R)-2-(6-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US11001575 (2021) BindingDB Entry DOI: 10.7270/Q2TB1B01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418266 ((2R)-2-(6-{5-chloro-2-[(1-methyl-1H- 1,2,3-triazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US11001575 (2021) BindingDB Entry DOI: 10.7270/Q2TB1B01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418305 ((2R)-2-(6-{5-chloro-2-[(2- methylpyrimidin-4-yl)am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US11001575 (2021) BindingDB Entry DOI: 10.7270/Q2TB1B01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418307 ((2R)-2-(6-}5-chloro-2-[(oxetan-3- yl)amino]pyrimid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD. US Patent | Assay Description Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... | US Patent US11001575 (2021) BindingDB Entry DOI: 10.7270/Q2TB1B01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2629 total ) | Next | Last >> |