

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50560224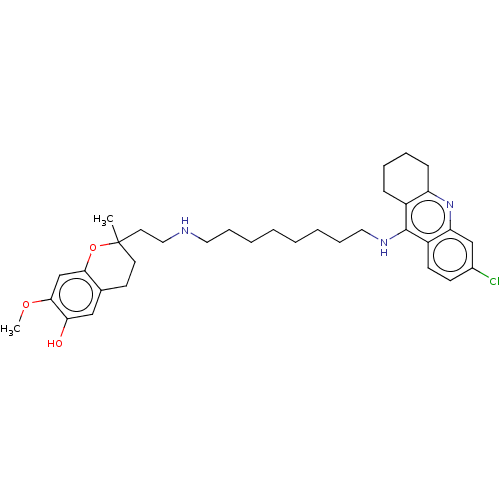 (CHEMBL4751100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of recombinant human AChE expressed in HEK293 cells assessed as dissociation constant for enzyme-inhibitor complex using varying lev... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00528 BindingDB Entry DOI: 10.7270/Q23N2733 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50560224 (CHEMBL4751100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of recombinant human AChE expressed in HEK293 cells assessed as dissociation constant for enzyme-substrate-inhibitor complex using v... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00528 BindingDB Entry DOI: 10.7270/Q23N2733 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of recombinant human AChE assessed as inhibition constant using acetylthiocholine iodide as substrate by Cornish-Bowden plot an... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50138279 (CHEMBL3752467) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of recombinant human AChE using acetylthiocholine iodide as substrate assessed as enzyme-inhibitor complex by Lineweaver-Burk d... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of recombinant human AChE assessed as dissociation constant for protein-substrate-compound complex using acetylthiocholine iodi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50138279 (CHEMBL3752467) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of recombinant human AChE using acetylthiocholine iodide as substrate assessed as enzyme-substrate-inhibitor complex by Linewea... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50138279 (CHEMBL3752467) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Torpedo californica AChE using ATCh as substrate preincubated for 90 mins followed by substrate addition by potentiometr... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50580216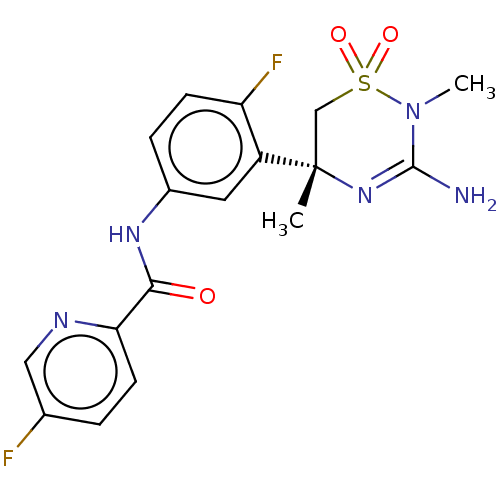 (MK-8931 | SCH 900931 | SCH-900931 | SCH900931 | VE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00931 BindingDB Entry DOI: 10.7270/Q2CF9V25 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50138279 (CHEMBL3752467) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of Torpedo californica AChE using ATCh as substrate preincubated for 90 mins followed by substrate addition by potentiometric ... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007801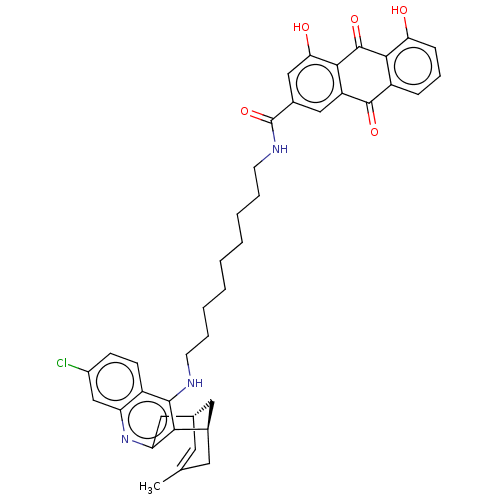 (CHEMBL3233832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate assessed as dissociation constant for enzyme-inhibitor complex by Li... | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007801 (CHEMBL3233832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate assessed as dissociation constant for enzyme-substrate-inhibitor com... | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50510823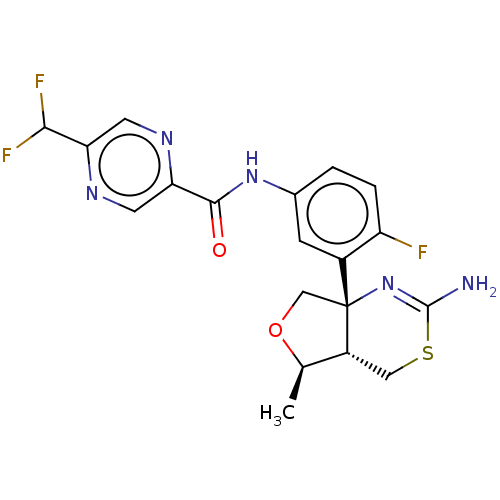 (E-2609 | E2609 | Elenbecestat) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00931 BindingDB Entry DOI: 10.7270/Q2CF9V25 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50062599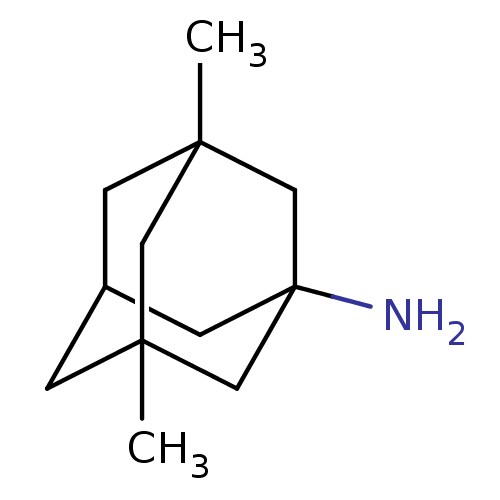 (3,5-Dimethyl-adamantan-1-ylamine | CHEMBL807 | EN3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant GluN1/GluN2B receptor (unknown origin) expressed in HEK293 cells by patch-clamp method | Eur J Med Chem 180: 613-626 (2019) Article DOI: 10.1016/j.ejmech.2019.07.051 BindingDB Entry DOI: 10.7270/Q2P55RRD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (Homo sapiens (Human)) | BDBM50502562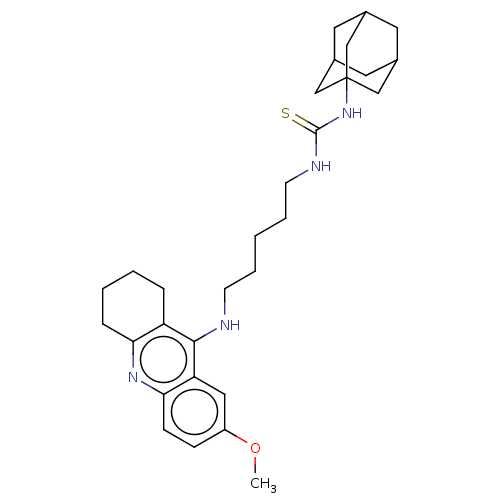 (CHEMBL4469066) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant GluN1/GluN2B receptor (unknown origin) expressed in HEK293 cells by patch-clamp method | Eur J Med Chem 180: 613-626 (2019) Article DOI: 10.1016/j.ejmech.2019.07.051 BindingDB Entry DOI: 10.7270/Q2P55RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50560224 (CHEMBL4751100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 15 mins followed by subst... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00528 BindingDB Entry DOI: 10.7270/Q23N2733 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50560225 (CHEMBL4785201) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 15 mins followed by subst... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00528 BindingDB Entry DOI: 10.7270/Q23N2733 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50502563 (CHEMBL4471659) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 20 mins followed by subst... | Eur J Med Chem 180: 613-626 (2019) Article DOI: 10.1016/j.ejmech.2019.07.051 BindingDB Entry DOI: 10.7270/Q2P55RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50579160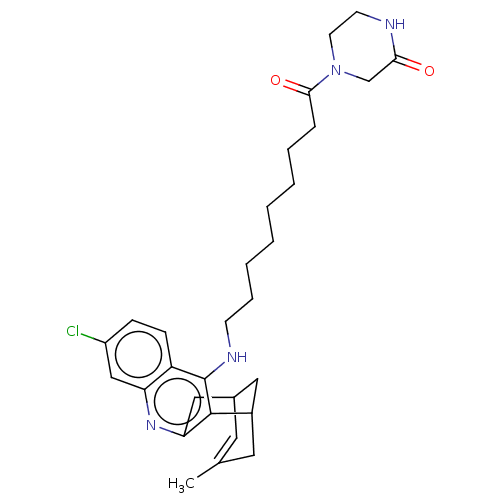 (CHEMBL4854913) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50560223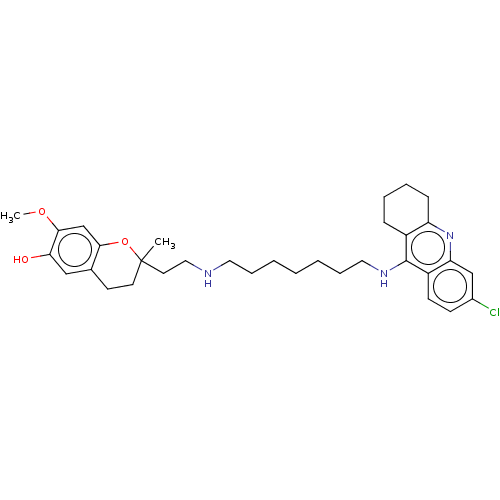 (CHEMBL4796461) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 15 mins followed by subst... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00528 BindingDB Entry DOI: 10.7270/Q23N2733 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138463 (CHEMBL3753957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138438 (CHEMBL3752969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236331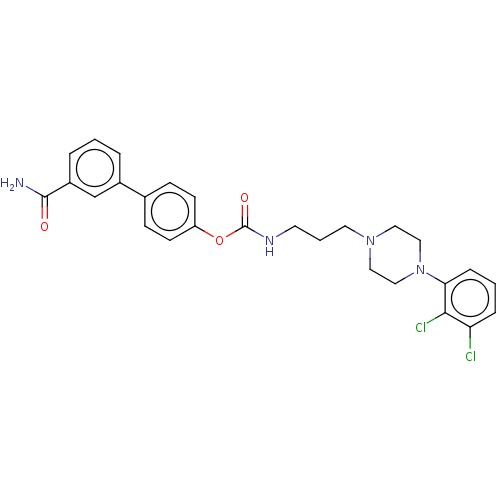 (CHEMBL4091498) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236333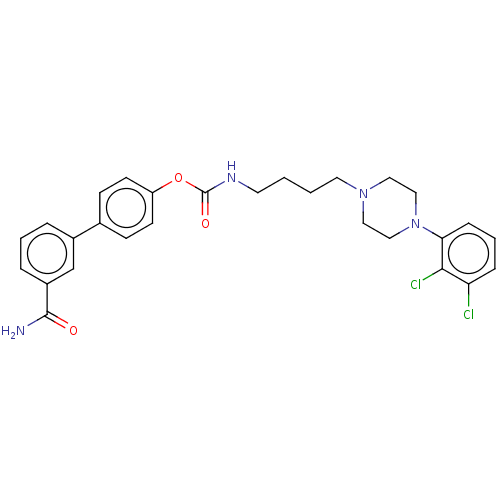 (CHEMBL4092052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236330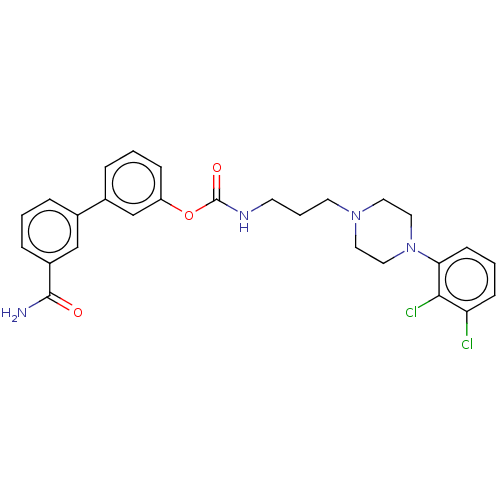 (CHEMBL4070196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM41542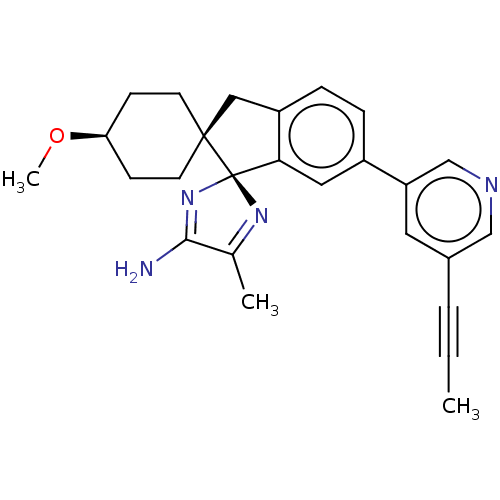 (US8865911, 122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00931 BindingDB Entry DOI: 10.7270/Q2CF9V25 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236343 (CHEMBL4086944) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50579161 (CHEMBL4848527) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379273 (CHEMBL1994202 | US9238626, (-)-Huprine Y HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50138279 (CHEMBL3752467) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236345 (CHEMBL4079093) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236340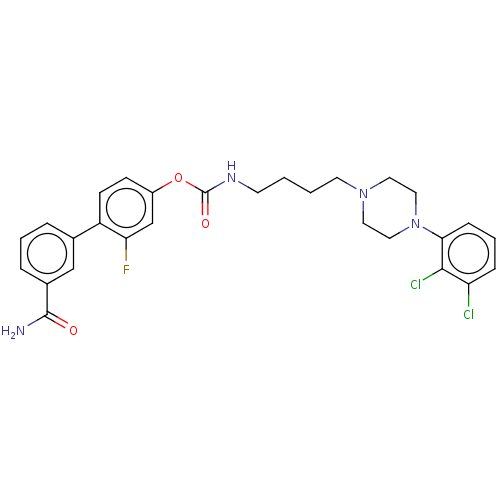 (CHEMBL4069565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236326 (CHEMBL4073966) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007795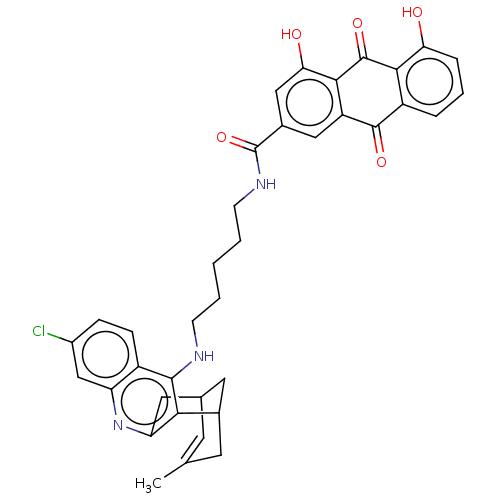 (CHEMBL3233826) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556958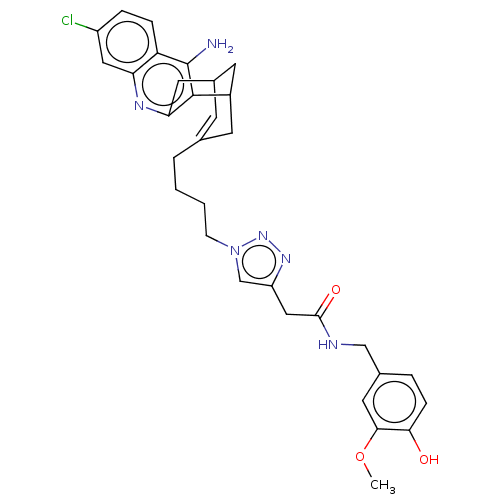 (CHEMBL4744528) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236342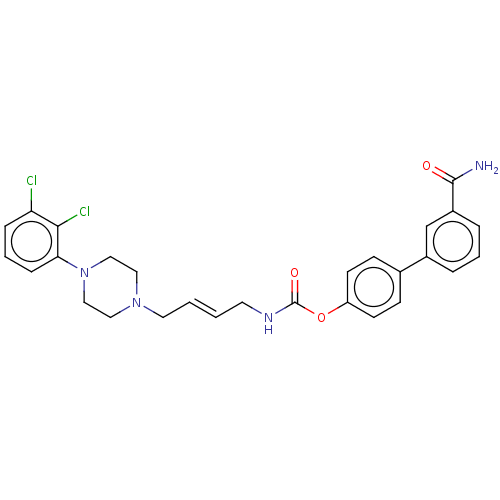 (CHEMBL4065510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138440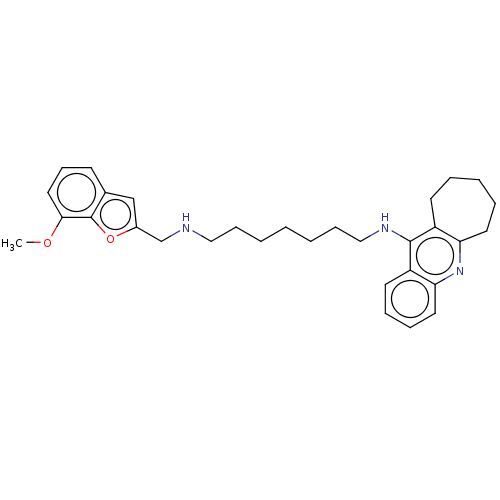 (CHEMBL3753405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50502565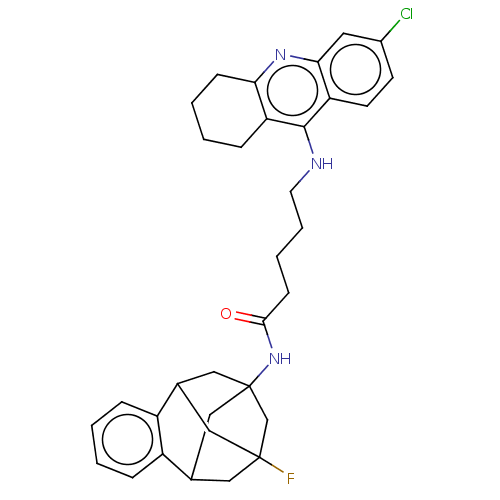 (CHEMBL4462369) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 20 mins followed by subst... | Eur J Med Chem 180: 613-626 (2019) Article DOI: 10.1016/j.ejmech.2019.07.051 BindingDB Entry DOI: 10.7270/Q2P55RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138437 (CHEMBL3752356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236327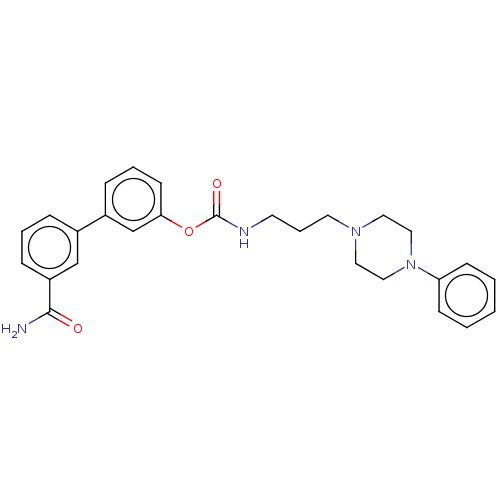 (CHEMBL4100735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Binding affinity against human Alpha-1a adrenergic receptor | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50579162 (CHEMBL4872514) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113779 BindingDB Entry DOI: 10.7270/Q2TB1BQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50502564 (CHEMBL4558495) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 20 mins followed by subst... | Eur J Med Chem 180: 613-626 (2019) Article DOI: 10.1016/j.ejmech.2019.07.051 BindingDB Entry DOI: 10.7270/Q2P55RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50502566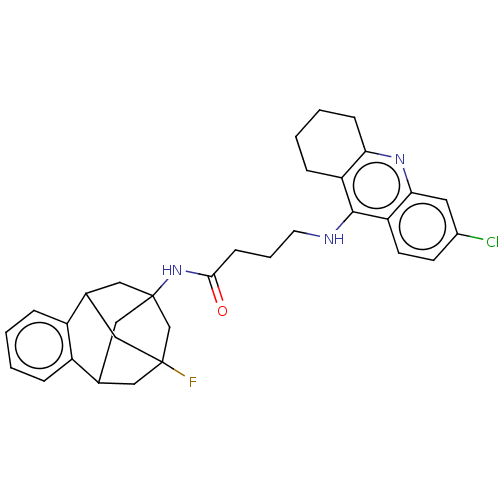 (CHEMBL4438626) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 20 mins followed by subst... | Eur J Med Chem 180: 613-626 (2019) Article DOI: 10.1016/j.ejmech.2019.07.051 BindingDB Entry DOI: 10.7270/Q2P55RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007796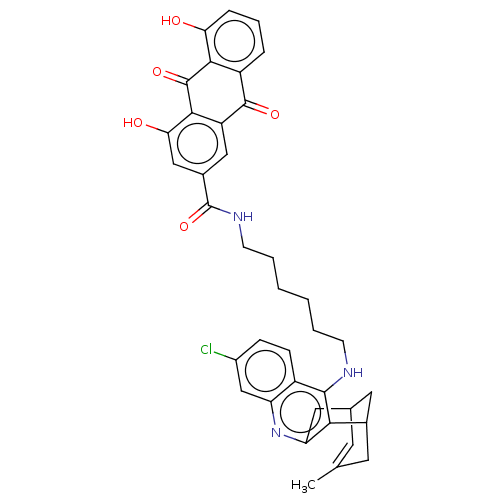 (CHEMBL3233827) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50236334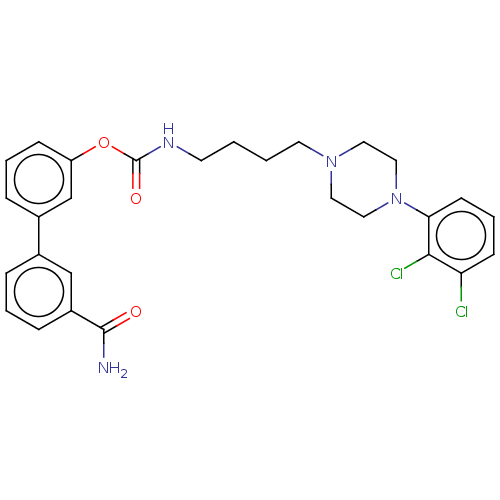 (CHEMBL4071240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bologna Curated by ChEMBL | Assay Description Inhibition of human FAAH1 expressed in HEK293 cell membrane-enriched lysate using AMC arachidonyl amide as substrate preincubated for 50 mins followe... | J Med Chem 60: 2287-2304 (2017) Article DOI: 10.1021/acs.jmedchem.6b01578 BindingDB Entry DOI: 10.7270/Q29S1T9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50231951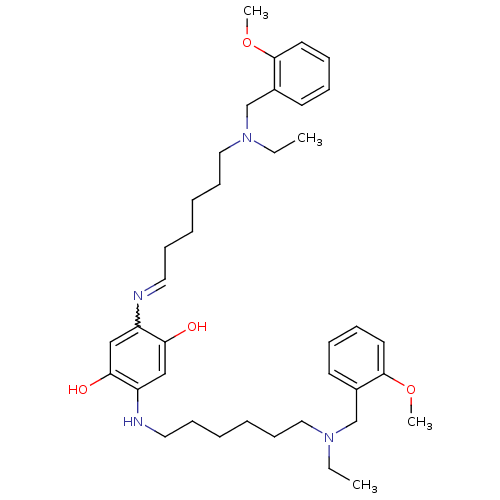 (2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's assay | Bioorg Med Chem Lett 23: 6254-8 (2013) Article DOI: 10.1016/j.bmcl.2013.09.091 BindingDB Entry DOI: 10.7270/Q22F7RDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 645 total ) | Next | Last >> |