 Found 756 hits with Last Name = 'debnath' and Initial = 'b'
Found 756 hits with Last Name = 'debnath' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinal dehydrogenase 2
(Homo sapiens (Human)) | BDBM50459608
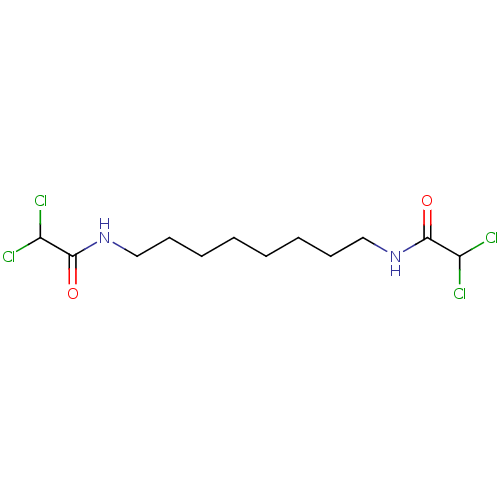
(CHEBI:90441 | CHEMBL3276621)Show InChI InChI=1S/C12H20Cl4N2O2/c13-9(14)11(19)17-7-5-3-1-2-4-6-8-18-12(20)10(15)16/h9-10H,1-8H2,(H,17,19)(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A2 |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldehyde dehydrogenase family 1 member A3
(Homo sapiens (Human)) | BDBM50459608

(CHEBI:90441 | CHEMBL3276621)Show InChI InChI=1S/C12H20Cl4N2O2/c13-9(14)11(19)17-7-5-3-1-2-4-6-8-18-12(20)10(15)16/h9-10H,1-8H2,(H,17,19)(H,18,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 261 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A3 |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50459608

(CHEBI:90441 | CHEMBL3276621)Show InChI InChI=1S/C12H20Cl4N2O2/c13-9(14)11(19)17-7-5-3-1-2-4-6-8-18-12(20)10(15)16/h9-10H,1-8H2,(H,17,19)(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50459601
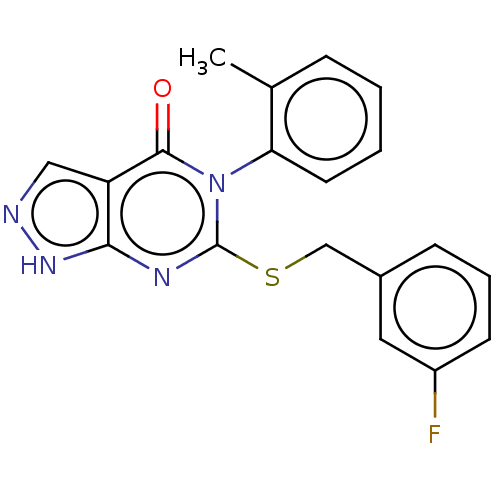
(CHEMBL4217738)Show SMILES Cc1ccccc1-n1c(SCc2cccc(F)c2)nc2[nH]ncc2c1=O |(15.24,-30.22,;16.57,-30.99,;17.9,-30.22,;19.24,-30.99,;19.24,-32.53,;17.9,-33.3,;16.57,-32.52,;15.24,-33.28,;15.24,-34.82,;16.58,-35.59,;16.57,-37.13,;17.91,-37.91,;17.9,-39.45,;19.23,-40.22,;20.57,-39.45,;20.56,-37.9,;21.9,-37.13,;19.23,-37.14,;13.91,-35.59,;12.59,-34.82,;11.12,-35.31,;10.21,-34.07,;11.11,-32.82,;12.58,-33.28,;13.91,-32.51,;13.92,-30.96,)| Show InChI InChI=1S/C19H15FN4OS/c1-12-5-2-3-8-16(12)24-18(25)15-10-21-23-17(15)22-19(24)26-11-13-6-4-7-14(20)9-13/h2-10H,11H2,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human recombinant ALDH1A1 assessed as reduction of NAD(P)H formation using varying levels of NAD+ |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393461
(CHEMBL2159938)Show SMILES CC(C)c1ccc(CN(Cc2ccccc2)C(c2nnnn2C2CCCC2)c2cc3cc(C)ccc3[nH]c2=O)cc1 Show InChI InChI=1S/C34H38N6O/c1-23(2)27-16-14-26(15-17-27)22-39(21-25-9-5-4-6-10-25)32(33-36-37-38-40(33)29-11-7-8-12-29)30-20-28-19-24(3)13-18-31(28)35-34(30)41/h4-6,9-10,13-20,23,29,32H,7-8,11-12,21-22H2,1-3H3,(H,35,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
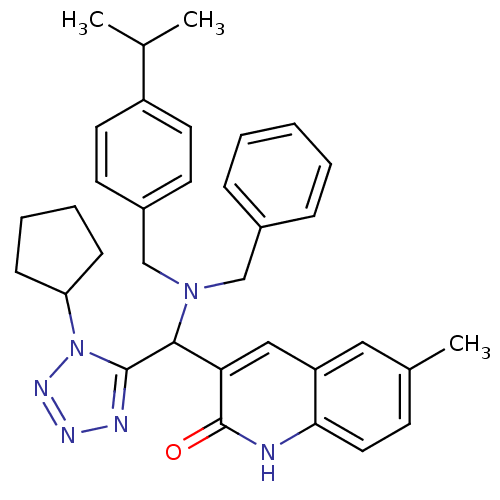
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393496
(CHEMBL2159935)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N1CCCC2(CCCCC2)C1)c1nnnn1C1CCCO1 Show InChI InChI=1S/C26H34N6O2/c1-18-8-9-21-19(15-18)16-20(25(33)27-21)23(24-28-29-30-32(24)22-7-5-14-34-22)31-13-6-12-26(17-31)10-3-2-4-11-26/h8-9,15-16,22-23H,2-7,10-14,17H2,1H3,(H,27,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393463
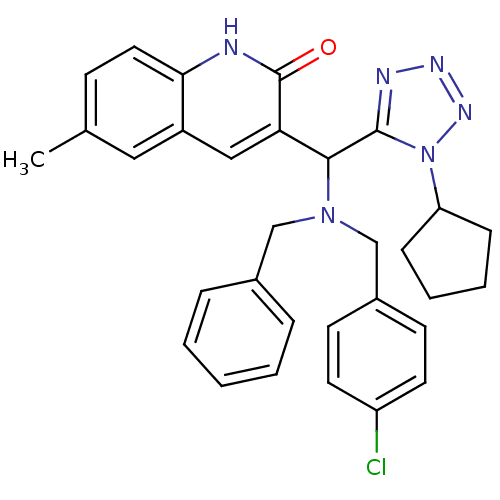
(CHEMBL2159940)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N(Cc1ccccc1)Cc1ccc(Cl)cc1)c1nnnn1C1CCCC1 Show InChI InChI=1S/C31H31ClN6O/c1-21-11-16-28-24(17-21)18-27(31(39)33-28)29(30-34-35-36-38(30)26-9-5-6-10-26)37(19-22-7-3-2-4-8-22)20-23-12-14-25(32)15-13-23/h2-4,7-8,11-18,26,29H,5-6,9-10,19-20H2,1H3,(H,33,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393461

(CHEMBL2159938)Show SMILES CC(C)c1ccc(CN(Cc2ccccc2)C(c2nnnn2C2CCCC2)c2cc3cc(C)ccc3[nH]c2=O)cc1 Show InChI InChI=1S/C34H38N6O/c1-23(2)27-16-14-26(15-17-27)22-39(21-25-9-5-4-6-10-25)32(33-36-37-38-40(33)29-11-7-8-12-29)30-20-28-19-24(3)13-18-31(28)35-34(30)41/h4-6,9-10,13-20,23,29,32H,7-8,11-12,21-22H2,1-3H3,(H,35,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393462

(CHEMBL2159939)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N1CCN(CC1)C(c1ccccc1)c1ccccc1)c1nnnn1C1CCCC1 Show InChI InChI=1S/C34H37N7O/c1-24-16-17-30-27(22-24)23-29(34(42)35-30)32(33-36-37-38-41(33)28-14-8-9-15-28)40-20-18-39(19-21-40)31(25-10-4-2-5-11-25)26-12-6-3-7-13-26/h2-7,10-13,16-17,22-23,28,31-32H,8-9,14-15,18-21H2,1H3,(H,35,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM46264

(3-[2-azaspiro[5.5]undecan-2-yl-(1-cyclopentyl-1,2,...)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N1CCCC2(CCCCC2)C1)c1nnnn1C1CCCC1 Show InChI InChI=1S/C27H36N6O/c1-19-10-11-23-20(16-19)17-22(26(34)28-23)24(25-29-30-31-33(25)21-8-3-4-9-21)32-15-7-14-27(18-32)12-5-2-6-13-27/h10-11,16-17,21,24H,2-9,12-15,18H2,1H3,(H,28,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50353441
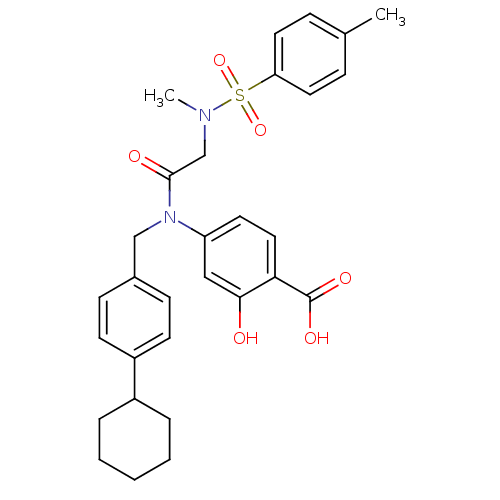
(CHEMBL1829786)Show SMILES CN(CC(=O)N(Cc1ccc(cc1)C1CCCCC1)c1ccc(C(O)=O)c(O)c1)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C30H34N2O6S/c1-21-8-15-26(16-9-21)39(37,38)31(2)20-29(34)32(25-14-17-27(30(35)36)28(33)18-25)19-22-10-12-24(13-11-22)23-6-4-3-5-7-23/h8-18,23,33H,3-7,19-20H2,1-2H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of STAT3 SH2 domain assessed as inhibition of STAT3 SH2-phosphotyrosine interaction after 15 mins by fluorescence polarization assay |
J Med Chem 55: 6645-68 (2012)
Article DOI: 10.1021/jm300207s
BindingDB Entry DOI: 10.7270/Q2CJ8FKK |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50382459
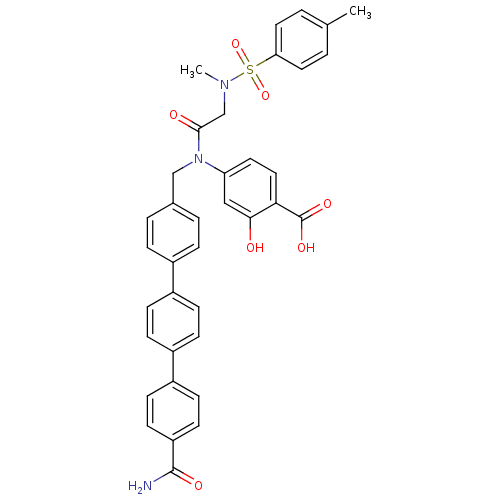
(CHEMBL2023986 | US10196373, Compound 27NH)Show SMILES CN(CC(=O)N(Cc1ccc(cc1)-c1ccc(cc1)-c1ccc(cc1)C(N)=O)c1ccc(C(O)=O)c(O)c1)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C37H33N3O7S/c1-24-3-18-32(19-4-24)48(46,47)39(2)23-35(42)40(31-17-20-33(37(44)45)34(41)21-31)22-25-5-7-26(8-6-25)27-9-11-28(12-10-27)29-13-15-30(16-14-29)36(38)43/h3-21,41H,22-23H2,1-2H3,(H2,38,43)(H,44,45) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of STAT3 SH2 domain assessed as inhibition of STAT3 SH2-phosphotyrosine interaction after 15 mins by fluorescence polarization assay |
J Med Chem 55: 6645-68 (2012)
Article DOI: 10.1021/jm300207s
BindingDB Entry DOI: 10.7270/Q2CJ8FKK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393496

(CHEMBL2159935)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N1CCCC2(CCCCC2)C1)c1nnnn1C1CCCO1 Show InChI InChI=1S/C26H34N6O2/c1-18-8-9-21-19(15-18)16-20(25(33)27-21)23(24-28-29-30-32(24)22-7-5-14-34-22)31-13-6-12-26(17-31)10-3-2-4-11-26/h8-9,15-16,22-23H,2-7,10-14,17H2,1H3,(H,27,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50396178
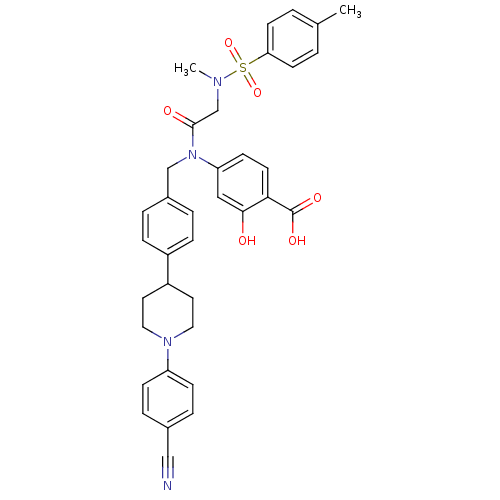
(CHEMBL2172014)Show SMILES CN(CC(=O)N(Cc1ccc(cc1)C1CCN(CC1)c1ccc(cc1)C#N)c1ccc(C(O)=O)c(O)c1)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C36H36N4O6S/c1-25-3-14-32(15-4-25)47(45,46)38(2)24-35(42)40(31-13-16-33(36(43)44)34(41)21-31)23-27-5-9-28(10-6-27)29-17-19-39(20-18-29)30-11-7-26(22-37)8-12-30/h3-16,21,29,41H,17-20,23-24H2,1-2H3,(H,43,44) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of STAT3 SH2 domain assessed as inhibition of STAT3 SH2-phosphotyrosine interaction after 15 mins by fluorescence polarization assay |
J Med Chem 55: 6645-68 (2012)
Article DOI: 10.1021/jm300207s
BindingDB Entry DOI: 10.7270/Q2CJ8FKK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393462

(CHEMBL2159939)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N1CCN(CC1)C(c1ccccc1)c1ccccc1)c1nnnn1C1CCCC1 Show InChI InChI=1S/C34H37N7O/c1-24-16-17-30-27(22-24)23-29(34(42)35-30)32(33-36-37-38-41(33)28-14-8-9-15-28)40-20-18-39(19-21-40)31(25-10-4-2-5-11-25)26-12-6-3-7-13-26/h2-7,10-13,16-17,22-23,28,31-32H,8-9,14-15,18-21H2,1H3,(H,35,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50382461
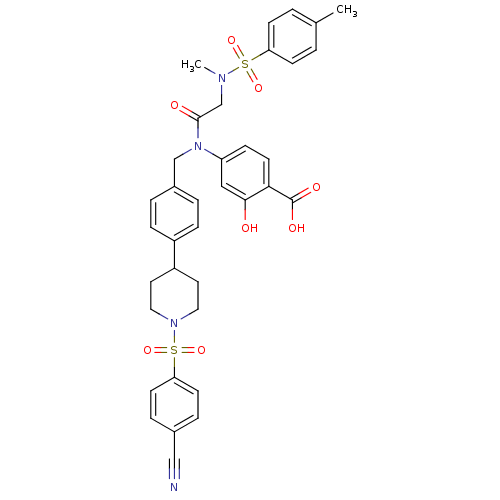
(CHEMBL2023987 | US10196373, Compound 27KG)Show SMILES CN(CC(=O)N(Cc1ccc(cc1)C1CCN(CC1)S(=O)(=O)c1ccc(cc1)C#N)c1ccc(C(O)=O)c(O)c1)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C36H36N4O8S2/c1-25-3-12-31(13-4-25)49(45,46)38(2)24-35(42)40(30-11-16-33(36(43)44)34(41)21-30)23-27-5-9-28(10-6-27)29-17-19-39(20-18-29)50(47,48)32-14-7-26(22-37)8-15-32/h3-16,21,29,41H,17-20,23-24H2,1-2H3,(H,43,44) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of STAT3 SH2 domain assessed as inhibition of STAT3 SH2-phosphotyrosine interaction after 15 mins by fluorescence polarization assay |
J Med Chem 55: 6645-68 (2012)
Article DOI: 10.1021/jm300207s
BindingDB Entry DOI: 10.7270/Q2CJ8FKK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50393463

(CHEMBL2159940)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N(Cc1ccccc1)Cc1ccc(Cl)cc1)c1nnnn1C1CCCC1 Show InChI InChI=1S/C31H31ClN6O/c1-21-11-16-28-24(17-21)18-27(31(39)33-28)29(30-34-35-36-38(30)26-9-5-6-10-26)37(19-22-7-3-2-4-8-22)20-23-12-14-25(32)15-13-23/h2-4,7-8,11-18,26,29H,5-6,9-10,19-20H2,1H3,(H,33,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
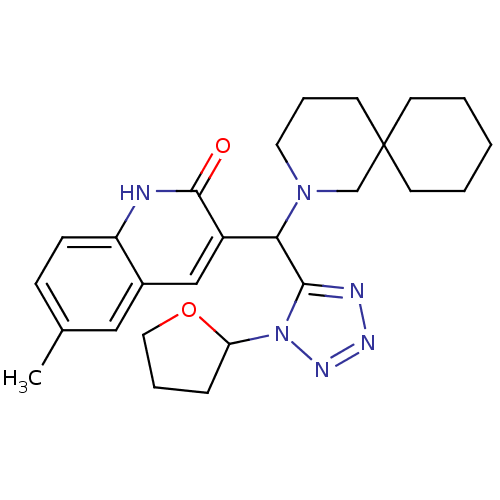
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM50335895
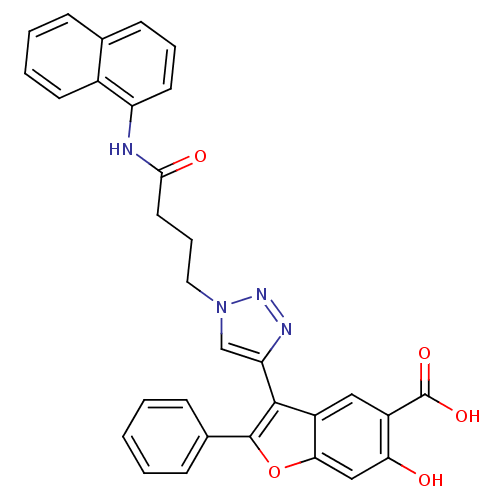
(6-hydroxy-3-(1-(4-(naphthalen-1-ylamino)-4-oxobuty...)Show SMILES OC(=O)c1cc2c(-c3cn(CCCC(=O)Nc4cccc5ccccc45)nn3)c(oc2cc1O)-c1ccccc1 Show InChI InChI=1S/C31H24N4O5/c36-26-17-27-23(16-22(26)31(38)39)29(30(40-27)20-9-2-1-3-10-20)25-18-35(34-33-25)15-7-14-28(37)32-24-13-6-11-19-8-4-5-12-21(19)24/h1-6,8-13,16-18,36H,7,14-15H2,(H,32,37)(H,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 22
(Homo sapiens (Human)) | BDBM46264

(3-[2-azaspiro[5.5]undecan-2-yl-(1-cyclopentyl-1,2,...)Show SMILES Cc1ccc2[nH]c(=O)c(cc2c1)C(N1CCCC2(CCCCC2)C1)c1nnnn1C1CCCC1 Show InChI InChI=1S/C27H36N6O/c1-19-10-11-23-20(16-19)17-22(26(34)28-23)24(25-29-30-31-33(25)21-8-3-4-9-21)32-15-7-14-27(18-32)12-5-2-6-13-27/h10-11,16-17,21,24H,2-9,12-15,18H2,1H3,(H,28,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of LYP expressed in Escherichia coli BL21 using increasing levels of ARLIED-NEpYTAREG substrate |
J Med Chem 54: 1640-54 (2011)
Article DOI: 10.1021/jm101202j
BindingDB Entry DOI: 10.7270/Q2RN38Z0 |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM50396179

(CHEMBL2172013)Show SMILES Cc1ccc(cc1)S(=O)(=O)OCC(=O)N(Cc1ccc(cc1)C1CCCCC1)c1ccc(C(O)=O)c(O)c1 Show InChI InChI=1S/C29H31NO7S/c1-20-7-14-25(15-8-20)38(35,36)37-19-28(32)30(24-13-16-26(29(33)34)27(31)17-24)18-21-9-11-23(12-10-21)22-5-3-2-4-6-22/h7-17,22,31H,2-6,18-19H2,1H3,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of STAT3 SH2 domain assessed as inhibition of STAT3 SH2-phosphotyrosine interaction after 15 mins by fluorescence polarization assay |
J Med Chem 55: 6645-68 (2012)
Article DOI: 10.1021/jm300207s
BindingDB Entry DOI: 10.7270/Q2CJ8FKK |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 3
(Homo sapiens (Human)) | BDBM20283
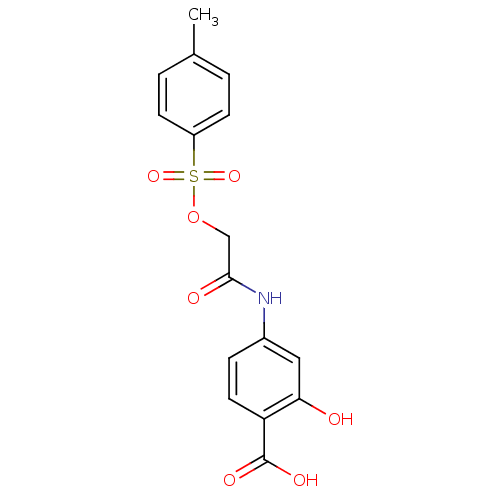
(2-hydroxy-4-(2-{[(4-methylbenzene)sulfonyl]oxy}ace...)Show SMILES Cc1ccc(cc1)S(=O)(=O)OCC(=O)Nc1ccc(C(O)=O)c(O)c1 Show InChI InChI=1S/C16H15NO7S/c1-10-2-5-12(6-3-10)25(22,23)24-9-15(19)17-11-4-7-13(16(20)21)14(18)8-11/h2-8,18H,9H2,1H3,(H,17,19)(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California
Curated by ChEMBL
| Assay Description
Inhibition of STAT3 SH2 domain assessed as inhibition of STAT3 SH2-phosphotyrosine interaction after 15 mins by fluorescence polarization assay |
J Med Chem 55: 6645-68 (2012)
Article DOI: 10.1021/jm300207s
BindingDB Entry DOI: 10.7270/Q2CJ8FKK |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090867

((2R,3S,4R,5R)-2-(aminomethyl)-5-(4-chloro-5-iodo-7...)Show SMILES NC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(Cl)ncnc12 |r| Show InChI InChI=1S/C11H12ClIN4O3/c12-9-6-4(13)2-17(10(6)16-3-15-9)11-8(19)7(18)5(1-14)20-11/h2-3,5,7-8,11,18-19H,1,14H2/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090843
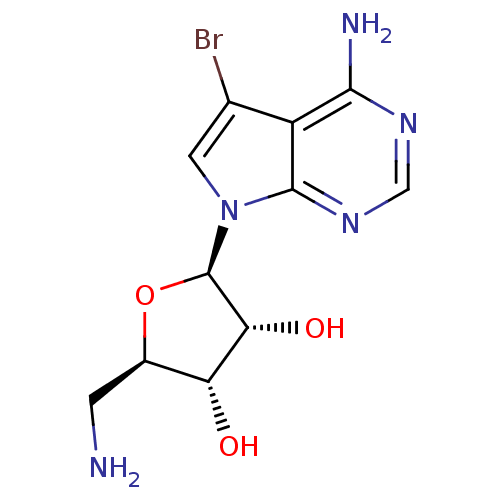
((1R,5S)-2-((3R,4aR)-4-Amino-5-bromo-pyrrolo[2,3-d]...)Show SMILES NC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(Br)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H14BrN5O3/c12-4-2-17(10-6(4)9(14)15-3-16-10)11-8(19)7(18)5(1-13)20-11/h2-3,5,7-8,11,18-19H,1,13H2,(H2,14,15,16)/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090862
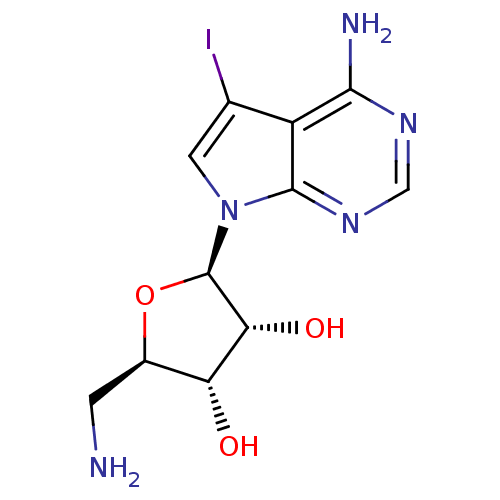
((1R,5S)-2-((3R,4aR)-4-Amino-5-iodo-pyrrolo[2,3-d]p...)Show SMILES NC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H14IN5O3/c12-4-2-17(10-6(4)9(14)15-3-16-10)11-8(19)7(18)5(1-13)20-11/h2-3,5,7-8,11,18-19H,1,13H2,(H2,14,15,16)/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090844

((2R,3R,4S,5R)-2-(4-Chloro-5-iodo-pyrrolo[2,3-d]pyr...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(Cl)ncnc12 |r| Show InChI InChI=1S/C11H11ClIN3O3/c1-4-7(17)8(18)11(19-4)16-2-5(13)6-9(12)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090854
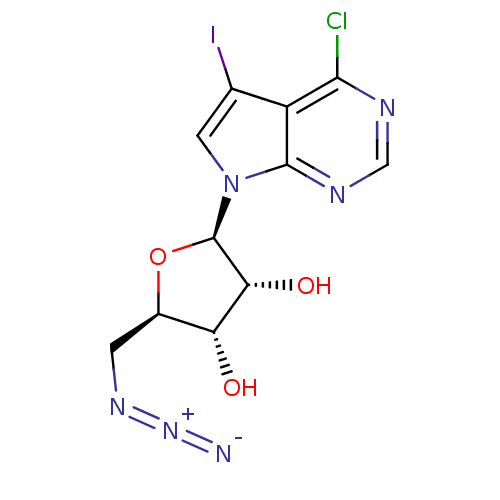
((2R,3S,4R,5R)-2-(azidomethyl)-5-(4-chloro-5-iodo-7...)Show SMILES O[C@@H]1[C@@H](CN=[N+]=[N-])O[C@H]([C@@H]1O)n1cc(I)c2c(Cl)ncnc12 |r| Show InChI InChI=1S/C11H10ClIN6O3/c12-9-6-4(13)2-19(10(6)16-3-15-9)11-8(21)7(20)5(22-11)1-17-18-14/h2-3,5,7-8,11,20-21H,1H2/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM14486
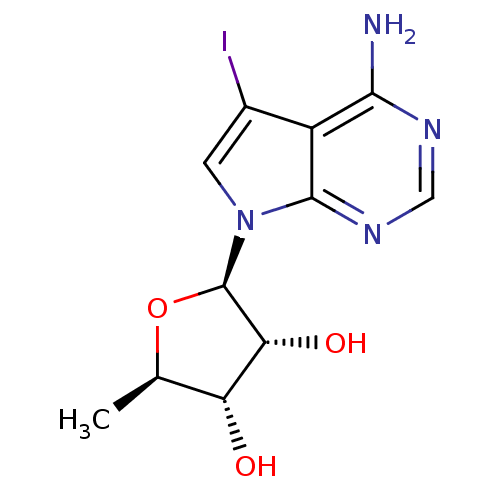
((2R,3R,4S,5R)-2-(2-amino-9-iodo-3,5,7-triazabicycl...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13IN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinal dehydrogenase 2
(Homo sapiens (Human)) | BDBM50567384
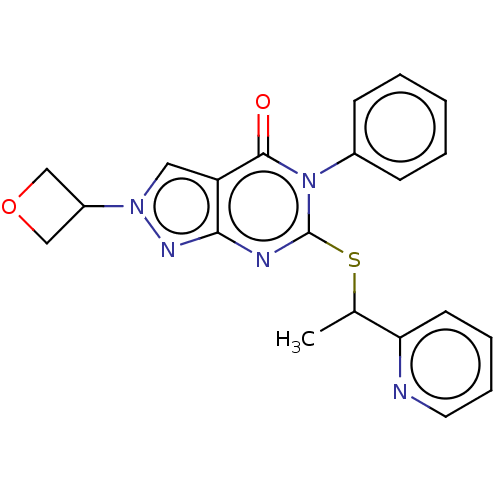
(CHEMBL4849586)Show SMILES CC(Sc1nc2nn(cc2c(=O)n1-c1ccccc1)C1COC1)c1ccccn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALDH1A2 assessed as NADH formation using propionaldehyde as substrate by spectrophotometery |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113060
BindingDB Entry DOI: 10.7270/Q2N58R5M |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090853
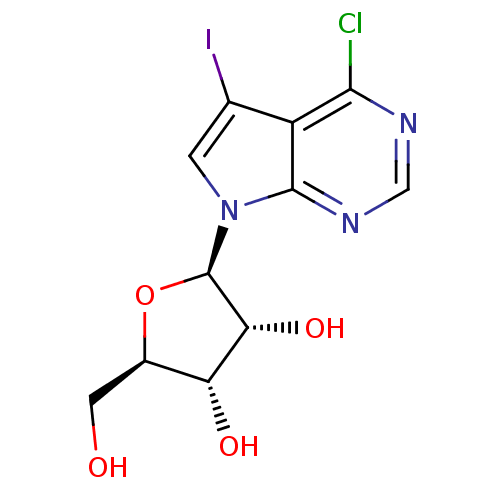
((2R,3R,4S,5R)-2-(4-Chloro-5-iodo-pyrrolo[2,3-d]pyr...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(Cl)ncnc12 |r| Show InChI InChI=1S/C11H11ClIN3O4/c12-9-6-4(13)1-16(10(6)15-3-14-9)11-8(19)7(18)5(2-17)20-11/h1,3,5,7-8,11,17-19H,2H2/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50042349
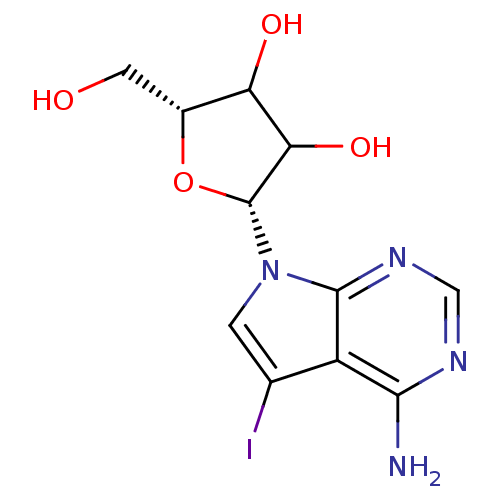
(2-(4-Amino-5-iodo-pyrrolo[2,3-d]pyrimidin-7-yl)-5-...)Show SMILES Nc1ncnc2n(cc(I)c12)[C@@H]1O[C@H](CO)C(O)C1O Show InChI InChI=1S/C11H13IN4O4/c12-4-1-16(10-6(4)9(13)14-3-15-10)11-8(19)7(18)5(2-17)20-11/h1,3,5,7-8,11,17-19H,2H2,(H2,13,14,15)/t5-,7?,8?,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase family 1 member A3
(Homo sapiens (Human)) | BDBM50567363
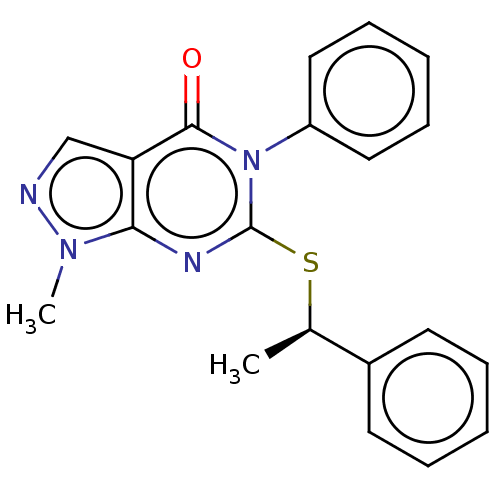
(CHEMBL4876602)Show SMILES C[C@@H](Sc1nc2n(C)ncc2c(=O)n1-c1ccccc1)c1ccccc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALDH1A3 assessed as NADH formation using propionaldehyde as substrate by spectrophotometery |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113060
BindingDB Entry DOI: 10.7270/Q2N58R5M |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090863

((2R,3R,4S,5R)-2-(4-Amino-5-iodo-pyrrolo[2,3-d]pyri...)Show SMILES Nc1ncnc2n(cc(I)c12)[C@@H]1O[C@H](CN=[N+]=[N-])[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12IN7O3/c12-4-2-19(10-6(4)9(13)15-3-16-10)11-8(21)7(20)5(22-11)1-17-18-14/h2-3,5,7-8,11,20-21H,1H2,(H2,13,15,16)/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Retinal dehydrogenase 2
(Homo sapiens (Human)) | BDBM50567363

(CHEMBL4876602)Show SMILES C[C@@H](Sc1nc2n(C)ncc2c(=O)n1-c1ccccc1)c1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALDH1A2 assessed as NADH formation using propionaldehyde as substrate by spectrophotometery |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113060
BindingDB Entry DOI: 10.7270/Q2N58R5M |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090847
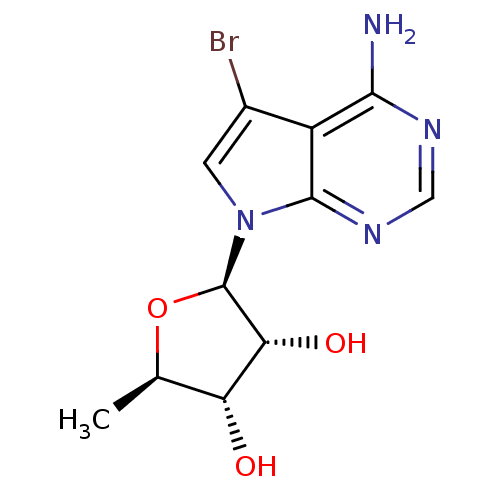
((2R,3R,4S,5R)-2-(4-Amino-5-bromo-pyrrolo[2,3-d]pyr...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(Br)c2c(N)ncnc12 |r| Show InChI InChI=1S/C11H13BrN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090855

((2R,3R,4S,5R)-2-(5-Iodo-4-methylsulfanyl-pyrrolo[2...)Show SMILES CSc1ncnc2n(cc(I)c12)[C@@H]1O[C@H](C)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H14IN3O3S/c1-5-8(17)9(18)12(19-5)16-3-6(13)7-10(16)14-4-15-11(7)20-2/h3-5,8-9,12,17-18H,1-2H3/t5-,8-,9-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090852

((2R,3R,4S,5R)-2-(5-Bromo-4-chloro-pyrrolo[2,3-d]py...)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(Br)c2c(Cl)ncnc12 |r| Show InChI InChI=1S/C11H11BrClN3O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3/t4-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase family 1 member A3
(Homo sapiens (Human)) | BDBM50567386
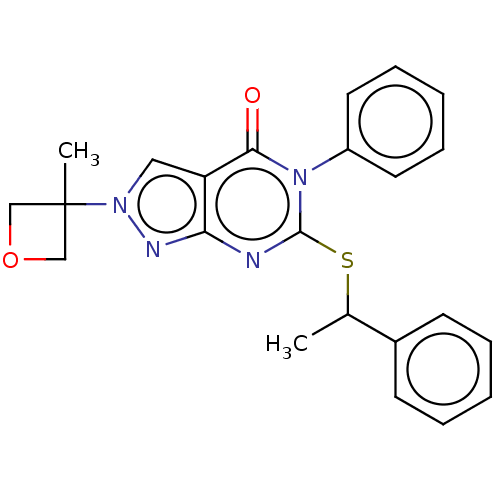
(CHEMBL4861872)Show SMILES CC(Sc1nc2nn(cc2c(=O)n1-c1ccccc1)C1(C)COC1)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALDH1A3 assessed as NADH formation using propionaldehyde as substrate by spectrophotometery |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113060
BindingDB Entry DOI: 10.7270/Q2N58R5M |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50076742
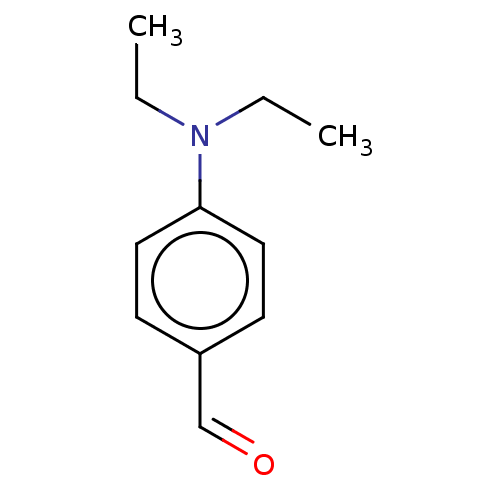
(CHEBI:86194 | CHEMBL3416563)Show InChI InChI=1S/C11H15NO/c1-3-12(4-2)11-7-5-10(9-13)6-8-11/h5-9H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | |
Retinal dehydrogenase 2
(Homo sapiens (Human)) | BDBM50567385
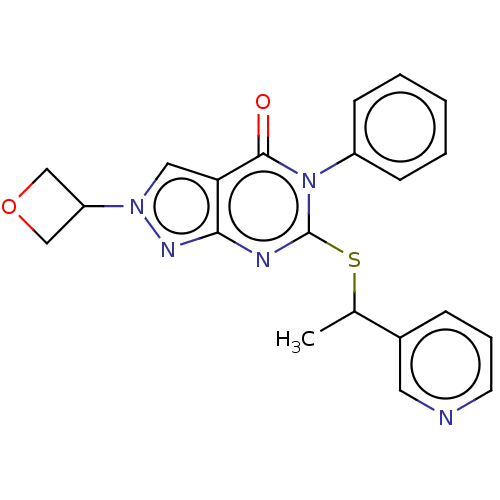
(CHEMBL4846203)Show SMILES CC(Sc1nc2nn(cc2c(=O)n1-c1ccccc1)C1COC1)c1cccnc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALDH1A2 assessed as NADH formation using propionaldehyde as substrate by spectrophotometery |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113060
BindingDB Entry DOI: 10.7270/Q2N58R5M |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase family 1 member A3
(Homo sapiens (Human)) | BDBM50567382
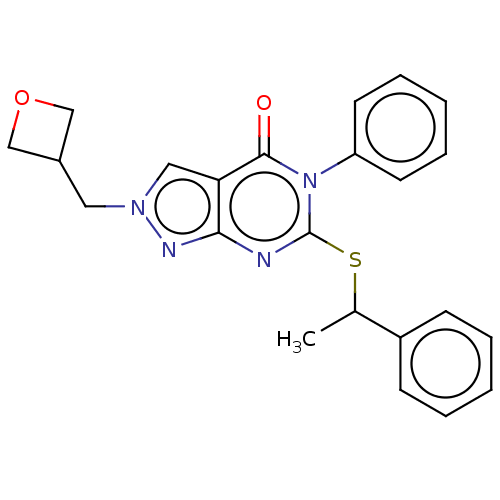
(CHEMBL4859904)Show SMILES CC(Sc1nc2nn(CC3COC3)cc2c(=O)n1-c1ccccc1)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALDH1A3 assessed as NADH formation using propionaldehyde as substrate by spectrophotometery |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113060
BindingDB Entry DOI: 10.7270/Q2N58R5M |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090864

((2R,3R,4S,5R)-2-(4-Amino-5-iodo-pyrrolo[2,3-d]pyri...)Show SMILES CC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cc(I)c2c(N)ncnc12 |r| Show InChI InChI=1S/C12H15IN4O3/c1-2-6-8(18)9(19)12(20-6)17-3-5(13)7-10(14)15-4-16-11(7)17/h3-4,6,8-9,12,18-19H,2H2,1H3,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090860
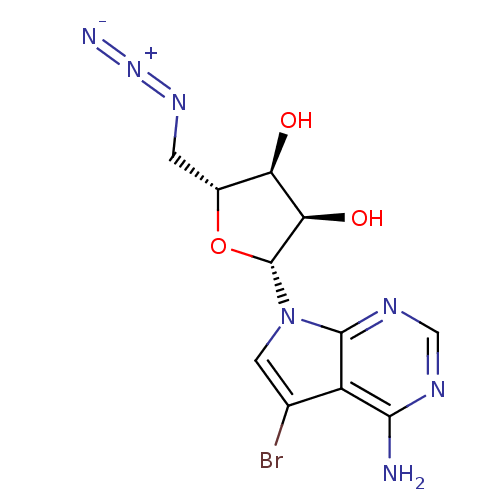
((2R,3R,4S,5R)-2-(4-Amino-5-bromo-pyrrolo[2,3-d]pyr...)Show SMILES Nc1ncnc2n(cc(Br)c12)[C@@H]1O[C@H](CN=[N+]=[N-])[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H12BrN7O3/c12-4-2-19(10-6(4)9(13)15-3-16-10)11-8(21)7(20)5(22-11)1-17-18-14/h2-3,5,7-8,11,20-21H,1H2,(H2,13,15,16)/t5-,7-,8-,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Retinal dehydrogenase 2
(Homo sapiens (Human)) | BDBM50567372
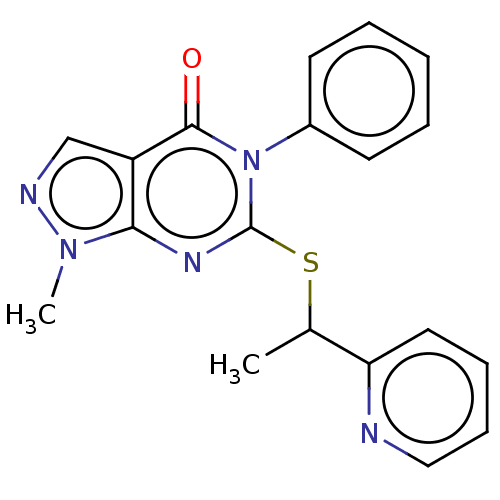
(CHEMBL4875157) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALDH1A2 assessed as NADH formation using propionaldehyde as substrate by spectrophotometery |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113060
BindingDB Entry DOI: 10.7270/Q2N58R5M |
More data for this
Ligand-Target Pair | |
Retinal dehydrogenase 2
(Homo sapiens (Human)) | BDBM50567383
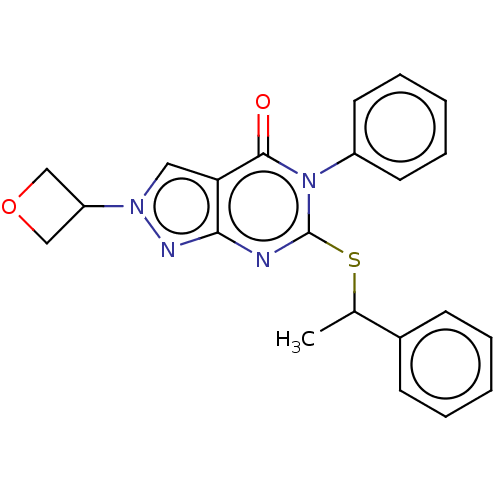
(CHEMBL4847114)Show SMILES CC(Sc1nc2nn(cc2c(=O)n1-c1ccccc1)C1COC1)c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALDH1A2 assessed as NADH formation using propionaldehyde as substrate by spectrophotometery |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113060
BindingDB Entry DOI: 10.7270/Q2N58R5M |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50567384

(CHEMBL4849586)Show SMILES CC(Sc1nc2nn(cc2c(=O)n1-c1ccccc1)C1COC1)c1ccccn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALDH1A1 assessed as NADH formation using propionaldehyde as substrate by spectrophotometery |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113060
BindingDB Entry DOI: 10.7270/Q2N58R5M |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50090866
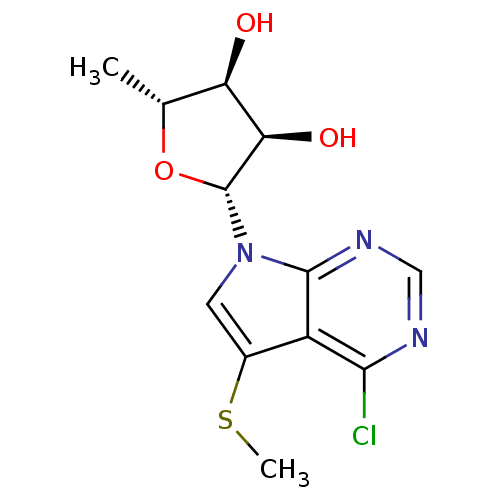
((2R,3R,4S,5R)-2-(4-Chloro-5-methylsulfanyl-pyrrolo...)Show SMILES CSc1cn([C@@H]2O[C@H](C)[C@@H](O)[C@H]2O)c2ncnc(Cl)c12 |r| Show InChI InChI=1S/C12H14ClN3O3S/c1-5-8(17)9(18)12(19-5)16-3-6(20-2)7-10(13)14-4-15-11(7)16/h3-5,8-9,12,17-18H,1-2H3/t5-,8-,9-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50567363

(CHEMBL4876602)Show SMILES C[C@@H](Sc1nc2n(C)ncc2c(=O)n1-c1ccccc1)c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALDH1A1 assessed as NADH formation using propionaldehyde as substrate by spectrophotometery |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113060
BindingDB Entry DOI: 10.7270/Q2N58R5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldehyde dehydrogenase family 1 member A3
(Homo sapiens (Human)) | BDBM50567362
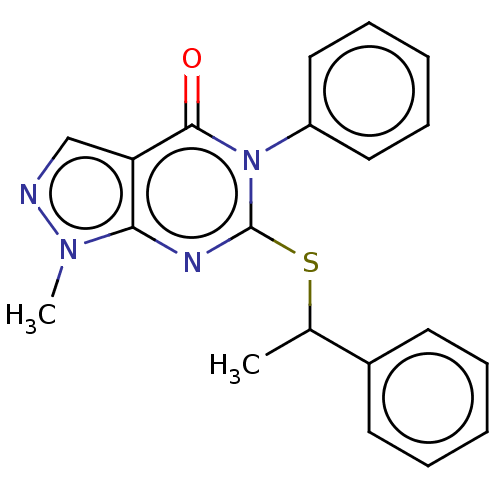
(CHEMBL4848258) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALDH1A3 assessed as NADH formation using propionaldehyde as substrate by spectrophotometery |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113060
BindingDB Entry DOI: 10.7270/Q2N58R5M |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50459588
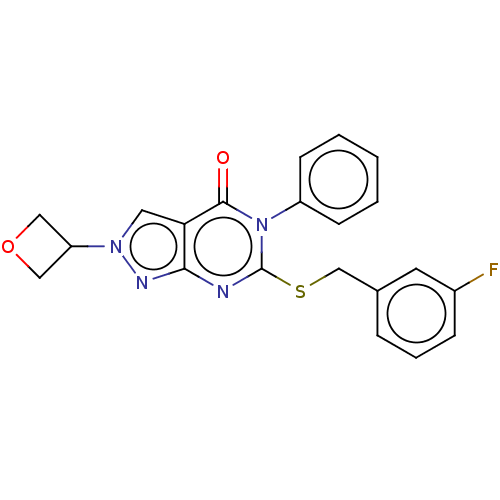
(CHEMBL4210115)Show SMILES Fc1cccc(CSc2nc3nn(cc3c(=O)n2-c2ccccc2)C2COC2)c1 Show InChI InChI=1S/C21H17FN4O2S/c22-15-6-4-5-14(9-15)13-29-21-23-19-18(10-25(24-19)17-11-28-12-17)20(27)26(21)16-7-2-1-3-8-16/h1-10,17H,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ALDH1A1 assessed as reduction in of NAD(P)H formation incubated for 2 mins by spectrophotometry |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50459603
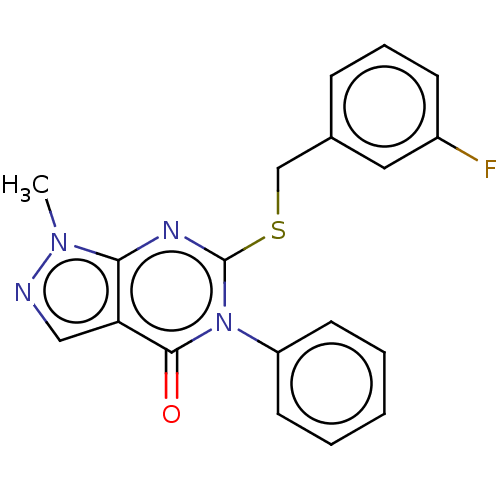
(CHEMBL4213331)Show InChI InChI=1S/C19H15FN4OS/c1-23-17-16(11-21-23)18(25)24(15-8-3-2-4-9-15)19(22-17)26-12-13-6-5-7-14(20)10-13/h2-11H,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ALDH1A1 assessed as reduction in of NAD(P)H formation incubated for 2 mins by spectrophotometry |
J Med Chem 61: 8754-8773 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00930
BindingDB Entry DOI: 10.7270/Q2V127F6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data