

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426742 (CHEMBL2322167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426763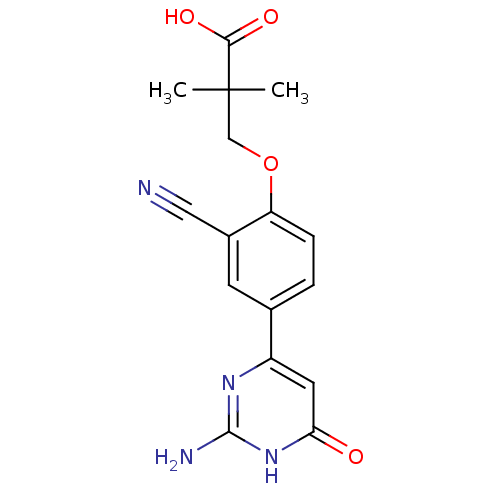 (CHEMBL2322147) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426761 (CHEMBL2322149) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426769 (CHEMBL2322169) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426768 (CHEMBL2322170) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426760 (CHEMBL2322150) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426759 (CHEMBL2322151) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426744 (CHEMBL2322165) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50436848 (CHEMBL2403617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using [14C]-oleoylCoA/diolein as substrate assessed as formation of [14C]triglyceride after 10 mins by scintillation counti... | Eur J Med Chem 65: 337-47 (2013) Article DOI: 10.1016/j.ejmech.2013.05.006 BindingDB Entry DOI: 10.7270/Q2QJ7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426764 (CHEMBL2322146) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426747 (CHEMBL2322162) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426746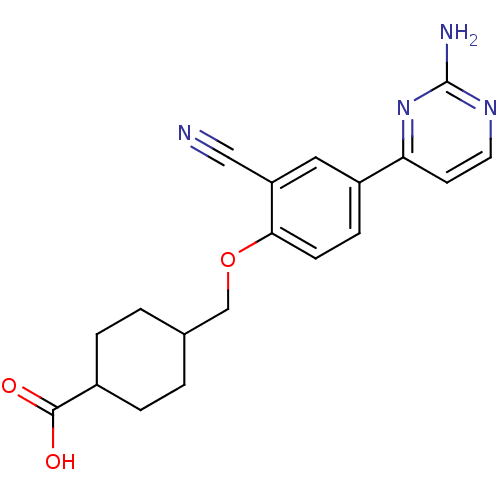 (CHEMBL2322163) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426767 (CHEMBL2322171) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426743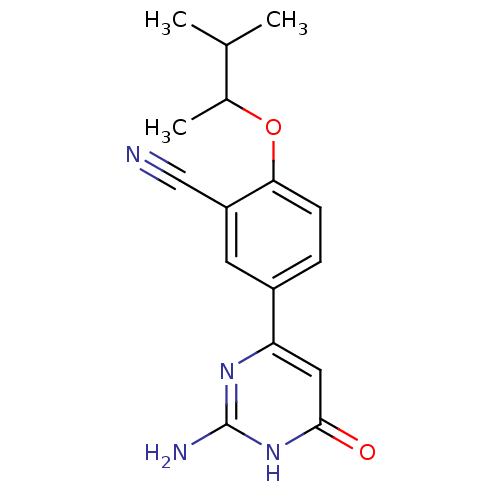 (CHEMBL2322166) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50354637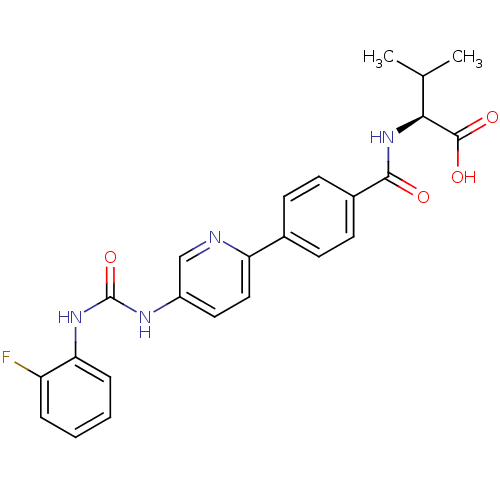 (CHEMBL1834440) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 assessed as formation of [14C]-triglyceride using [14C]oleoyl-CoA by liquid scintillation counting | Bioorg Med Chem Lett 21: 5812-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.109 BindingDB Entry DOI: 10.7270/Q28G8M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50354642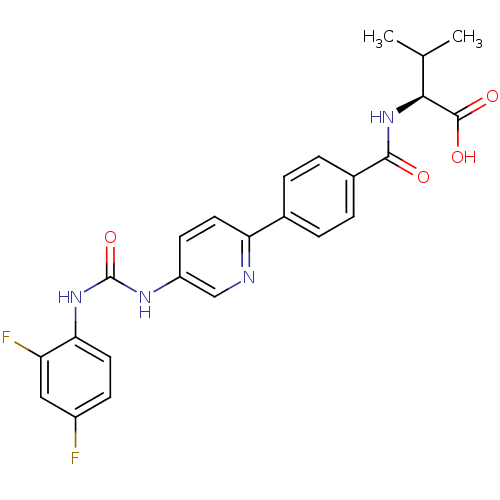 (CHEMBL1834204) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 assessed as formation of [14C]-triglyceride using [14C]oleoyl-CoA by liquid scintillation counting | Bioorg Med Chem Lett 21: 5812-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.109 BindingDB Entry DOI: 10.7270/Q28G8M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50005651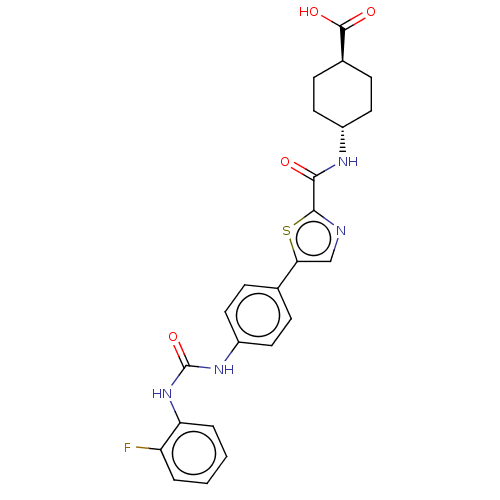 (CHEMBL3235427) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... | Eur J Med Chem 79: 203-15 (2014) Article DOI: 10.1016/j.ejmech.2014.03.077 BindingDB Entry DOI: 10.7270/Q23R0VCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426762 (CHEMBL2322148) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50436846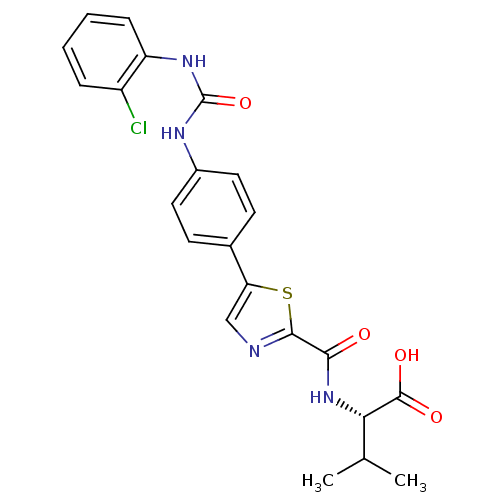 (CHEMBL2403620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using [14C]-oleoylCoA/diolein as substrate assessed as formation of [14C]triglyceride after 10 mins by scintillation counti... | Eur J Med Chem 65: 337-47 (2013) Article DOI: 10.1016/j.ejmech.2013.05.006 BindingDB Entry DOI: 10.7270/Q2QJ7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426741 (CHEMBL2322168) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50354639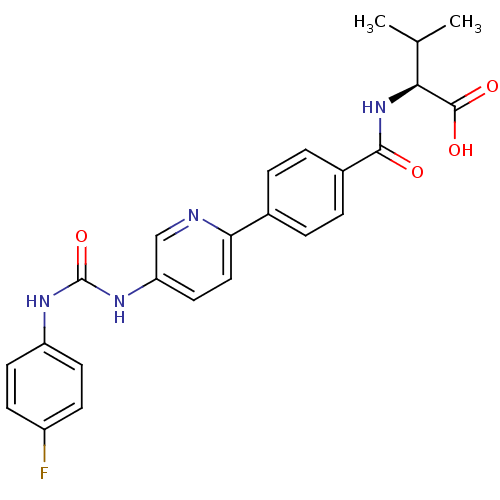 (CHEMBL1834201) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 assessed as formation of [14C]-triglyceride using [14C]oleoyl-CoA by liquid scintillation counting | Bioorg Med Chem Lett 21: 5812-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.109 BindingDB Entry DOI: 10.7270/Q28G8M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426765 (CHEMBL2322145) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50354638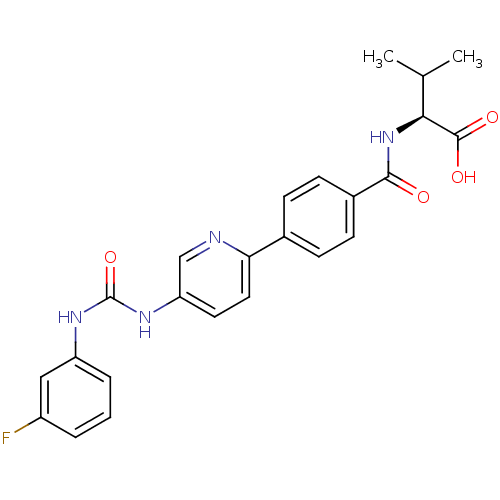 (CHEMBL1834200) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 assessed as formation of [14C]-triglyceride using [14C]oleoyl-CoA by liquid scintillation counting | Bioorg Med Chem Lett 21: 5812-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.109 BindingDB Entry DOI: 10.7270/Q28G8M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426758 (CHEMBL2322152) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426766 (CHEMBL2322144) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM20716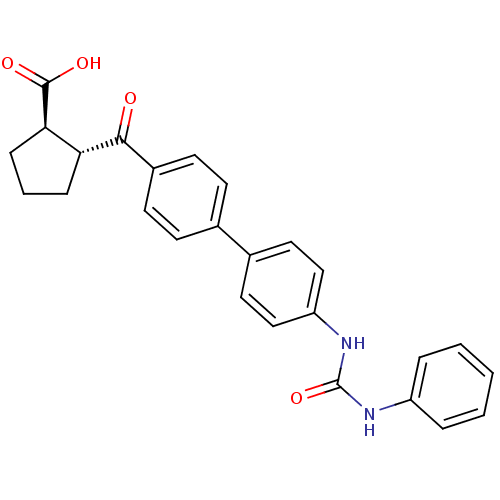 ((1R,2R)-2-[(4-{4-[(phenylcarbamoyl)amino]phenyl}ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in Sf9 cells assessed as formation of didecanoylglycerol product after 1 hr using 14C-decanoyl-CoA by beta scinti... | Bioorg Med Chem Lett 21: 5812-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.109 BindingDB Entry DOI: 10.7270/Q28G8M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM20716 ((1R,2R)-2-[(4-{4-[(phenylcarbamoyl)amino]phenyl}ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 assessed as formation of [14C]-triglyceride using [14C]oleoyl-CoA by liquid scintillation counting | Bioorg Med Chem Lett 21: 5812-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.109 BindingDB Entry DOI: 10.7270/Q28G8M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50402272 (CHEMBL2205509) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase using xanthine as substrate at 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 22: 7543-6 (2012) Article DOI: 10.1016/j.bmcl.2012.10.029 BindingDB Entry DOI: 10.7270/Q2445NNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50005652 (CHEMBL3235428) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... | Eur J Med Chem 79: 203-15 (2014) Article DOI: 10.1016/j.ejmech.2014.03.077 BindingDB Entry DOI: 10.7270/Q23R0VCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50436845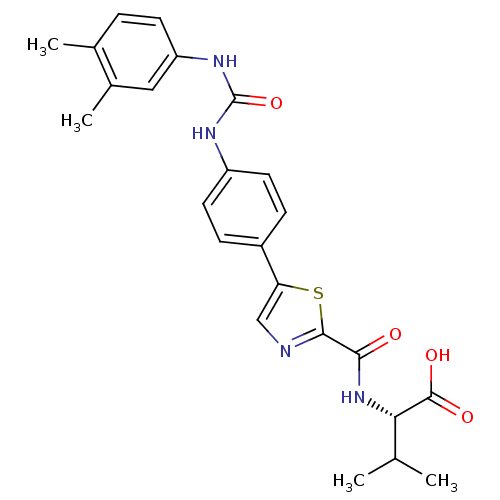 (CHEMBL2403621) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using [14C]-oleoylCoA/diolein as substrate assessed as formation of [14C]triglyceride after 10 mins by scintillation counti... | Eur J Med Chem 65: 337-47 (2013) Article DOI: 10.1016/j.ejmech.2013.05.006 BindingDB Entry DOI: 10.7270/Q2QJ7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50320491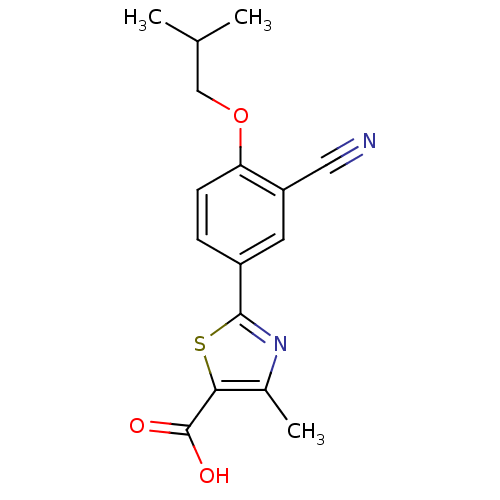 (2-(3-CYANO-4-ISOBUTOXY-PHENYL)-4-METHYL-5-THIAZOLE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50436847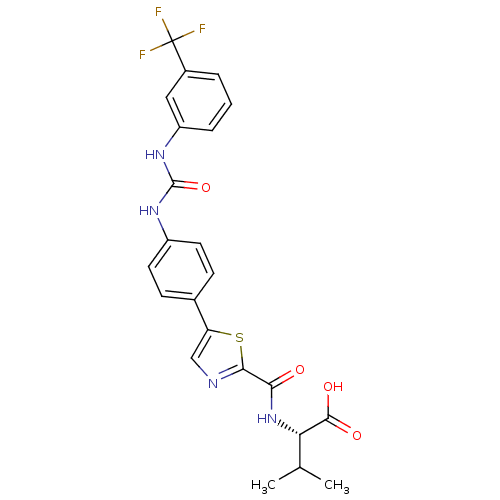 (CHEMBL2403619) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using [14C]-oleoylCoA/diolein as substrate assessed as formation of [14C]triglyceride after 10 mins by scintillation counti... | Eur J Med Chem 65: 337-47 (2013) Article DOI: 10.1016/j.ejmech.2013.05.006 BindingDB Entry DOI: 10.7270/Q2QJ7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50436844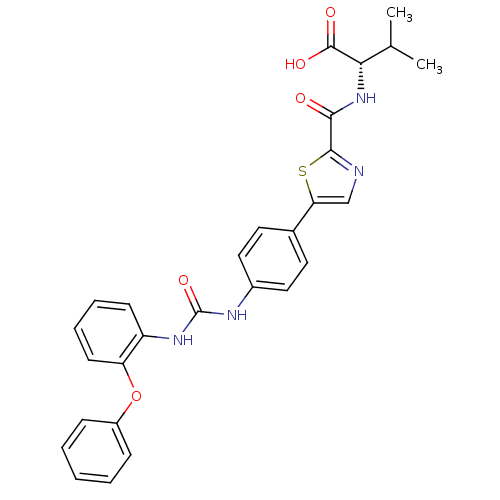 (CHEMBL2403622) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using [14C]-oleoylCoA/diolein as substrate assessed as formation of [14C]triglyceride after 10 mins by scintillation counti... | Eur J Med Chem 65: 337-47 (2013) Article DOI: 10.1016/j.ejmech.2013.05.006 BindingDB Entry DOI: 10.7270/Q2QJ7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50436842 (CHEMBL2403624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using [14C]-oleoylCoA/diolein as substrate assessed as formation of [14C]triglyceride after 10 mins by scintillation counti... | Eur J Med Chem 65: 337-47 (2013) Article DOI: 10.1016/j.ejmech.2013.05.006 BindingDB Entry DOI: 10.7270/Q2QJ7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50354641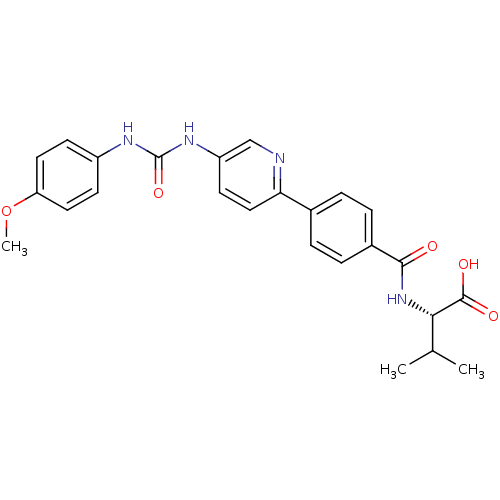 (CHEMBL1834203) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 assessed as formation of [14C]-triglyceride using [14C]oleoyl-CoA by liquid scintillation counting | Bioorg Med Chem Lett 21: 5812-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.109 BindingDB Entry DOI: 10.7270/Q28G8M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50005654 (CHEMBL3235430) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... | Eur J Med Chem 79: 203-15 (2014) Article DOI: 10.1016/j.ejmech.2014.03.077 BindingDB Entry DOI: 10.7270/Q23R0VCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50354631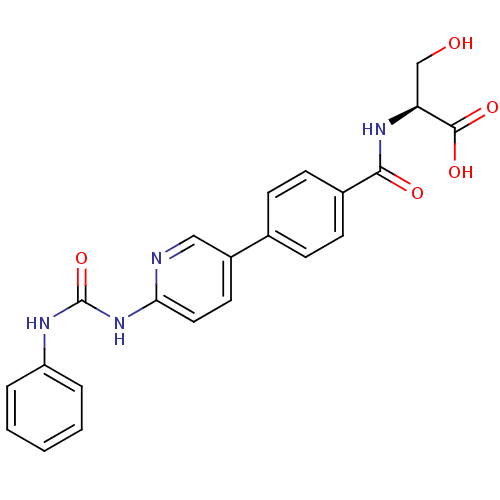 (CHEMBL1834433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 assessed as formation of [14C]-triglyceride using [14C]oleoyl-CoA by liquid scintillation counting | Bioorg Med Chem Lett 21: 5812-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.109 BindingDB Entry DOI: 10.7270/Q28G8M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50320491 (2-(3-CYANO-4-ISOBUTOXY-PHENYL)-4-METHYL-5-THIAZOLE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase using xanthine as substrate at 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 22: 7543-6 (2012) Article DOI: 10.1016/j.bmcl.2012.10.029 BindingDB Entry DOI: 10.7270/Q2445NNH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50354629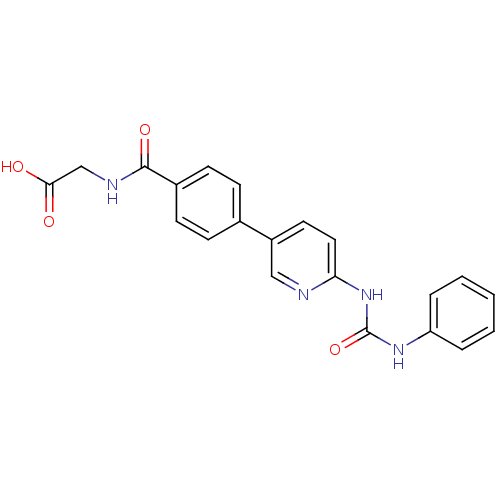 (CHEMBL1834431) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 assessed as formation of [14C]-triglyceride using [14C]oleoyl-CoA by liquid scintillation counting | Bioorg Med Chem Lett 21: 5812-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.109 BindingDB Entry DOI: 10.7270/Q28G8M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50354634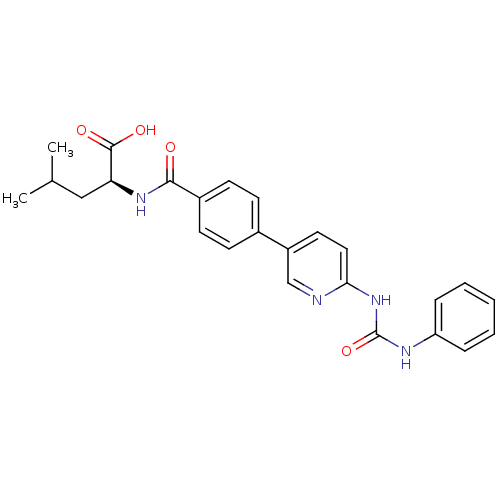 (CHEMBL1834436) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 assessed as formation of [14C]-triglyceride using [14C]oleoyl-CoA by liquid scintillation counting | Bioorg Med Chem Lett 21: 5812-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.109 BindingDB Entry DOI: 10.7270/Q28G8M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50005649 (CHEMBL3235425) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... | Eur J Med Chem 79: 203-15 (2014) Article DOI: 10.1016/j.ejmech.2014.03.077 BindingDB Entry DOI: 10.7270/Q23R0VCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50386149 (CHEMBL2042351) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 | Eur J Med Chem 65: 337-47 (2013) Article DOI: 10.1016/j.ejmech.2013.05.006 BindingDB Entry DOI: 10.7270/Q2QJ7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50436841 (CHEMBL2403625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using [14C]-oleoylCoA/diolein as substrate assessed as formation of [14C]triglyceride after 10 mins by scintillation counti... | Eur J Med Chem 65: 337-47 (2013) Article DOI: 10.1016/j.ejmech.2013.05.006 BindingDB Entry DOI: 10.7270/Q2QJ7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50005653 (CHEMBL3235429) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... | Eur J Med Chem 79: 203-15 (2014) Article DOI: 10.1016/j.ejmech.2014.03.077 BindingDB Entry DOI: 10.7270/Q23R0VCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50436839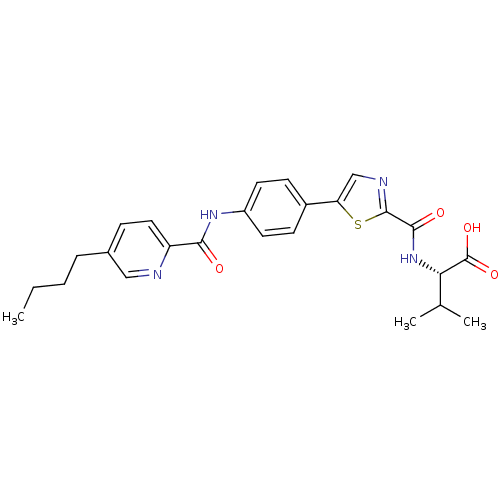 (CHEMBL2403627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using [14C]-oleoylCoA/diolein as substrate assessed as formation of [14C]triglyceride after 10 mins by scintillation counti... | Eur J Med Chem 65: 337-47 (2013) Article DOI: 10.1016/j.ejmech.2013.05.006 BindingDB Entry DOI: 10.7270/Q2QJ7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50426748 (CHEMBL2322161) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Healthcare Limited Curated by ChEMBL | Assay Description Inhibition of bovine xanthine oxidase-mediated uric acid production after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 23: 834-8 (2013) Article DOI: 10.1016/j.bmcl.2012.11.057 BindingDB Entry DOI: 10.7270/Q28W3FMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50354633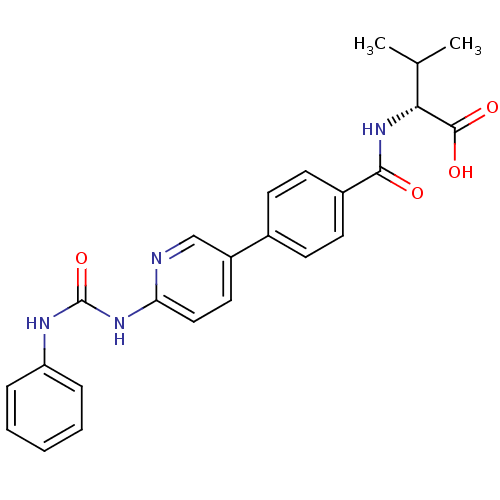 (CHEMBL1834435) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 assessed as formation of [14C]-triglyceride using [14C]oleoyl-CoA by liquid scintillation counting | Bioorg Med Chem Lett 21: 5812-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.109 BindingDB Entry DOI: 10.7270/Q28G8M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50005655 (CHEMBL3235431) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... | Eur J Med Chem 79: 203-15 (2014) Article DOI: 10.1016/j.ejmech.2014.03.077 BindingDB Entry DOI: 10.7270/Q23R0VCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50354636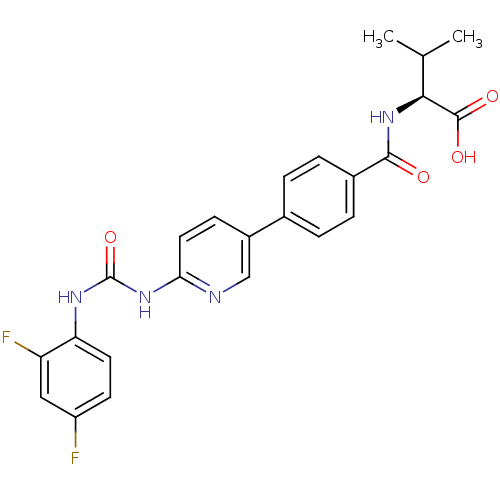 (CHEMBL1834438) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 assessed as formation of [14C]-triglyceride using [14C]oleoyl-CoA by liquid scintillation counting | Bioorg Med Chem Lett 21: 5812-7 (2011) Article DOI: 10.1016/j.bmcl.2011.07.109 BindingDB Entry DOI: 10.7270/Q28G8M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50436843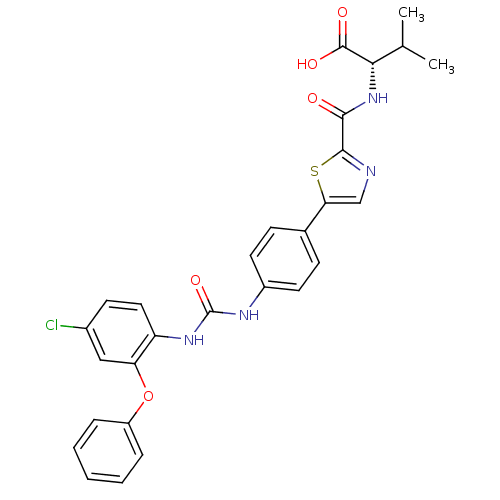 (CHEMBL2403623) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using [14C]-oleoylCoA/diolein as substrate assessed as formation of [14C]triglyceride after 10 mins by scintillation counti... | Eur J Med Chem 65: 337-47 (2013) Article DOI: 10.1016/j.ejmech.2013.05.006 BindingDB Entry DOI: 10.7270/Q2QJ7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 136 total ) | Next | Last >> |