 Found 181 hits with Last Name = 'dimaio' and Initial = 'j'
Found 181 hits with Last Name = 'dimaio' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50072528
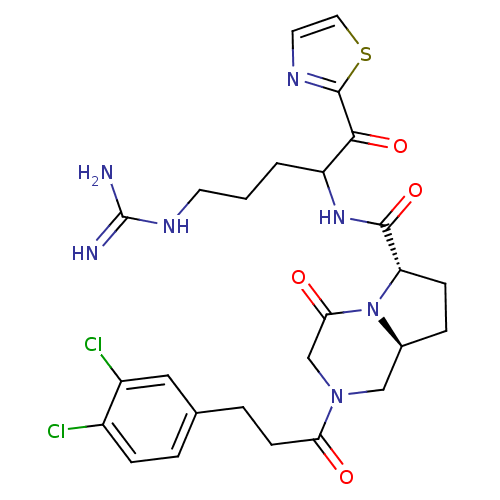
((6S,8aS)-2-[3-(3,4-Dichloro-phenyl)-propionyl]-4-o...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccc(Cl)c(Cl)c1)C(=O)c1nccs1 Show InChI InChI=1S/C26H31Cl2N7O4S/c27-17-6-3-15(12-18(17)28)4-8-21(36)34-13-16-5-7-20(35(16)22(37)14-34)24(39)33-19(2-1-9-32-26(29)30)23(38)25-31-10-11-40-25/h3,6,10-12,16,19-20H,1-2,4-5,7-9,13-14H2,(H,33,39)(H4,29,30,32)/t16-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004744

(CHEMBL2370453 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)CCCCC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O |wU:132.137,4.4,65.73,49.54,26.34,96.105,17.22,37.38,166.171,81.88,145.149,174.179,wD:107.108,75.79,57.62,8.13,154.158,114.125,136.140,2.2,(23.18,-7.9,;24.72,-8.01,;25.58,-6.74,;24.91,-5.35,;27.12,-6.85,;27.79,-8.23,;26.93,-9.51,;25.39,-9.4,;27.6,-10.89,;29.14,-11.01,;30,-9.73,;31.54,-9.84,;32.21,-11.23,;32.4,-8.57,;26.74,-12.17,;27.41,-13.55,;28.94,-13.67,;26.54,-14.83,;25.01,-14.72,;24.14,-15.99,;22.61,-15.88,;21.94,-14.5,;21.74,-17.16,;27.22,-16.22,;26.35,-17.49,;24.82,-17.38,;27.02,-18.88,;26.16,-20.15,;26.83,-21.54,;25.97,-22.81,;26.64,-24.2,;28.18,-24.31,;29.04,-23.03,;28.37,-21.65,;28.56,-18.99,;29.42,-17.71,;28.75,-16.33,;30.96,-17.82,;31.63,-19.21,;30.77,-20.48,;31.44,-21.87,;29.23,-20.37,;31.82,-16.55,;33.36,-16.66,;34.03,-18.04,;34.22,-15.38,;35.76,-15.49,;36.62,-14.22,;35.95,-12.83,;38.16,-14.33,;38.83,-15.72,;40.37,-15.83,;41.04,-17.21,;41.82,-15.3,;39.02,-13.06,;40.56,-13.17,;41.23,-14.55,;41.42,-11.89,;40.75,-10.51,;41.62,-9.23,;42.92,-8.42,;40.94,-7.85,;42.96,-12,;43.82,-10.73,;43.15,-9.34,;45.36,-10.84,;46.03,-12.22,;47.57,-12.34,;48.38,-13.64,;49.88,-13.27,;49.99,-11.74,;48.56,-11.16,;46.22,-9.56,;47.76,-9.67,;49.12,-10.4,;48.62,-8.4,;47.95,-7.01,;46.42,-6.9,;50.16,-8.51,;51.02,-7.24,;50.35,-5.85,;52.56,-7.35,;53.23,-8.73,;54.77,-8.84,;55.44,-10.23,;54.58,-11.5,;56.98,-10.34,;53.42,-6.07,;54.96,-6.18,;55.63,-7.57,;55.82,-4.91,;57.36,-5.02,;58.22,-3.74,;59.76,-3.86,;60.62,-2.58,;59.95,-1.19,;62.16,-2.69,;62.83,-4.08,;61.97,-5.35,;62.64,-6.74,;64.18,-6.85,;64.85,-8.23,;63.98,-9.51,;66.38,-8.34,;63.02,-1.42,;64.56,-1.53,;65.23,-2.91,;65.42,-.25,;64.9,1.2,;66.12,2.14,;67.39,1.28,;66.96,-.2,;67.91,-1.42,;67.33,-2.84,;69.43,-1.21,;70.01,.22,;71.54,.43,;72.48,-.79,;74.01,-.57,;74.59,.85,;73.64,2.07,;72.12,1.86,;70.38,-2.42,;71.9,-2.21,;72.85,-3.43,;73.21,-1.4,;27.98,-5.57,;29.52,-5.68,;27.31,-4.19,;25.8,-3.92,;25.58,-2.39,;26.97,-1.72,;28.04,-2.83,;29.56,-2.62,;30.51,-3.83,;30.14,-1.19,;31.67,-.98,;32.61,-2.2,;32.03,-3.62,;32.98,-4.84,;32.4,-6.27,;34.5,-4.63,;32.25,.45,;31.3,1.66,;33.77,.66,;34.35,2.08,;33.41,3.3,;31.88,3.09,;30.94,4.3,;31.52,5.73,;29.41,4.09,;35.88,2.29,;36.83,1.08,;36.46,3.72,;37.99,3.93,;38.93,2.72,;40.46,2.93,;41.4,1.71,;42.93,1.92,;43.51,3.35,;45.03,3.56,;42.56,4.56,;41.04,4.35,;38.57,5.36,;37.62,6.57,;40.09,5.57,;40.67,7,;39.73,8.21,;40.31,9.64,;39.36,10.85,;41.83,9.85,;42.2,7.21,;43.14,5.99,;42.78,8.63,;44.3,8.84,;44.88,10.27,;46.41,10.48,;46.99,11.91,;46.04,13.12,;48.51,12.12,;45.25,7.63,;44.67,6.2,;46.77,7.84,)| Show InChI InChI=1S/C115H162N28O40/c1-6-59(4)96(113(181)143-45-17-24-83(143)111(179)132-70(35-41-92(157)158)100(168)129-69(34-40-91(155)156)101(169)135-75(48-63-27-29-65(146)30-28-63)105(173)134-73(46-58(2)3)103(171)133-72(114(182)183)32-38-86(117)149)141-102(170)71(36-42-93(159)160)130-99(167)68(33-39-90(153)154)131-104(172)74(47-61-18-9-7-10-19-61)136-108(176)79(53-95(163)164)127-89(152)55-123-97(165)78(52-94(161)162)139-107(175)77(51-87(118)150)138-106(174)76(50-64-54-121-57-124-64)137-109(177)81(56-144)140-98(166)67(31-37-85(116)148)126-88(151)26-14-13-25-84(147)66(22-15-43-122-115(119)120)128-110(178)82-23-16-44-142(82)112(180)80(125-60(5)145)49-62-20-11-8-12-21-62/h7-12,18-21,27-30,54,57-59,66-83,96,144,146H,6,13-17,22-26,31-53,55-56H2,1-5H3,(H2,116,148)(H2,117,149)(H2,118,150)(H,121,124)(H,123,165)(H,125,145)(H,126,151)(H,127,152)(H,128,178)(H,129,168)(H,130,167)(H,131,172)(H,132,179)(H,133,171)(H,134,173)(H,135,169)(H,136,176)(H,137,177)(H,138,174)(H,139,175)(H,140,166)(H,141,170)(H,153,154)(H,155,156)(H,157,158)(H,159,160)(H,161,162)(H,163,164)(H,182,183)(H4,119,120,122)/t59-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,96-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072536
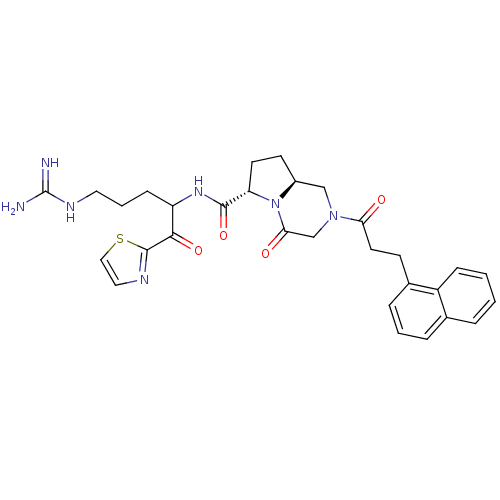
((6S,8aS)-2-(3-Naphthalen-1-yl-propionyl)-4-oxo-oct...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1cccc2ccccc12)C(=O)c1nccs1 Show InChI InChI=1S/C30H35N7O4S/c31-30(32)34-14-4-9-23(27(40)29-33-15-16-42-29)35-28(41)24-12-11-21-17-36(18-26(39)37(21)24)25(38)13-10-20-7-3-6-19-5-1-2-8-22(19)20/h1-3,5-8,15-16,21,23-24H,4,9-14,17-18H2,(H,35,41)(H4,31,32,34)/t21-,23?,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004739
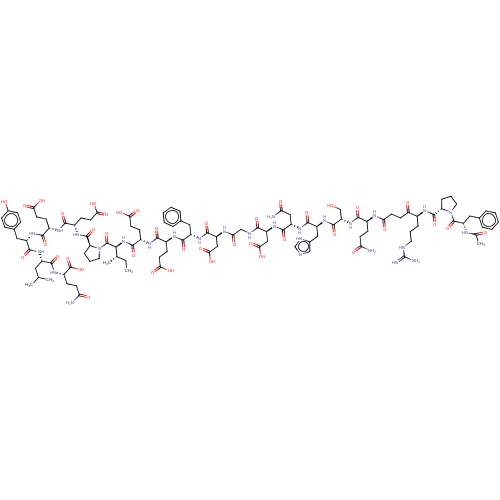
(CHEMBL2370455 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)CCC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O |wU:130.135,4.4,105.106,65.73,49.54,26.34,94.103,17.22,37.38,112.123,81.88,143.147,172.177,wD:75.79,57.62,8.13,152.156,164.169,134.138,2.2,(23.16,-12.23,;24.7,-12.29,;25.52,-10.99,;24.81,-9.62,;27.06,-11.05,;27.78,-12.41,;26.96,-13.71,;25.42,-13.65,;27.68,-15.07,;29.22,-15.13,;30.04,-13.83,;31.58,-13.89,;32.29,-15.25,;32.4,-12.59,;26.86,-16.38,;27.57,-17.74,;29.11,-17.8,;26.75,-19.04,;25.22,-18.98,;24.39,-20.29,;22.86,-20.23,;22.14,-18.86,;22.03,-21.53,;27.47,-20.4,;26.65,-21.71,;25.11,-21.65,;27.37,-23.07,;26.55,-24.37,;27.27,-25.74,;28.81,-25.79,;29.52,-27.16,;28.7,-28.46,;27.16,-28.4,;26.45,-27.04,;28.91,-23.13,;29.73,-21.83,;29.01,-20.46,;31.27,-21.89,;31.99,-23.25,;31.17,-24.55,;31.88,-25.91,;29.63,-24.49,;32.09,-20.58,;33.63,-20.64,;34.35,-22,;34.45,-19.34,;35.99,-19.4,;36.81,-18.09,;36.09,-16.73,;38.35,-18.15,;39.07,-19.52,;40.6,-19.58,;42.03,-19,;41.32,-20.94,;39.17,-16.85,;40.71,-16.91,;41.42,-18.27,;41.53,-15.61,;40.81,-14.24,;41.63,-12.94,;40.91,-11.58,;42.91,-12.08,;43.07,-15.67,;43.89,-14.36,;43.17,-13,;45.43,-14.42,;46.14,-15.78,;47.68,-15.84,;48.54,-17.12,;50.02,-16.7,;50.08,-15.17,;48.64,-14.63,;46.25,-13.12,;47.79,-13.18,;49.17,-13.86,;48.61,-11.88,;47.89,-10.51,;46.35,-10.45,;50.14,-11.93,;50.97,-10.63,;50.25,-9.27,;52.5,-10.69,;53.22,-12.05,;54.76,-12.11,;55.48,-13.47,;54.66,-14.78,;57.02,-13.53,;53.33,-9.39,;54.86,-9.45,;55.58,-10.81,;55.68,-8.14,;57.22,-8.2,;58.04,-6.9,;57.33,-5.54,;59.58,-6.96,;60.3,-8.32,;59.48,-9.62,;60.2,-10.99,;61.74,-11.05,;62.46,-12.41,;61.63,-13.71,;63.99,-12.47,;60.4,-5.66,;61.94,-5.72,;62.66,-7.08,;62.76,-4.41,;64.3,-4.31,;64.68,-2.82,;63.37,-2,;62.19,-2.98,;60.7,-2.6,;59.63,-3.71,;60.28,-1.12,;61.35,-.02,;60.93,1.46,;59.44,1.84,;59.02,3.32,;60.1,4.43,;61.59,4.05,;62.01,2.57,;58.79,-.74,;58.37,.74,;56.87,1.11,;58.59,2.26,;27.88,-9.74,;29.42,-9.8,;27.16,-8.38,;25.64,-8.16,;25.38,-6.64,;26.74,-5.93,;27.84,-7,;29.36,-6.74,;30.35,-7.92,;29.89,-5.29,;31.41,-5.03,;32.4,-6.21,;31.87,-7.66,;32.85,-8.84,;34.37,-8.58,;32.32,-10.29,;31.94,-3.58,;30.96,-2.4,;33.46,-3.32,;33.99,-1.88,;33.01,-.69,;31.49,-.96,;30.5,.23,;31.04,1.67,;28.99,-.03,;35.51,-1.62,;36.5,-2.8,;36.04,-.17,;37.56,.09,;38.55,-1.09,;40.06,-.83,;41.05,-2.01,;42.57,-1.75,;43.1,-.31,;44.62,-.04,;42.11,.88,;40.59,.62,;38.09,1.54,;37.11,2.72,;39.61,1.8,;40.14,3.24,;41.66,3.51,;42.64,2.32,;44.16,2.59,;43.06,.84,;39.16,4.43,;37.64,4.17,;39.69,5.87,;41.2,6.13,;41.74,7.58,;43.25,7.84,;43.79,9.29,;45.3,9.55,;42.8,10.47,;42.19,4.95,;42.61,3.47,;43.71,5.21,)| Show InChI InChI=1S/C113H158N28O40/c1-6-57(4)94(111(179)141-43-15-22-81(141)109(177)130-68(31-39-90(155)156)98(166)127-67(30-38-89(153)154)99(167)133-73(46-61-23-25-63(144)26-24-61)103(171)132-71(44-56(2)3)101(169)131-70(112(180)181)28-35-84(115)147)139-100(168)69(32-40-91(157)158)128-97(165)66(29-37-88(151)152)129-102(170)72(45-59-16-9-7-10-17-59)134-106(174)77(51-93(161)162)125-87(150)53-121-95(163)76(50-92(159)160)137-105(173)75(49-85(116)148)136-104(172)74(48-62-52-119-55-122-62)135-107(175)79(54-142)138-96(164)65(27-34-83(114)146)124-86(149)36-33-82(145)64(20-13-41-120-113(117)118)126-108(176)80-21-14-42-140(80)110(178)78(123-58(5)143)47-60-18-11-8-12-19-60/h7-12,16-19,23-26,52,55-57,64-81,94,142,144H,6,13-15,20-22,27-51,53-54H2,1-5H3,(H2,114,146)(H2,115,147)(H2,116,148)(H,119,122)(H,121,163)(H,123,143)(H,124,149)(H,125,150)(H,126,176)(H,127,166)(H,128,165)(H,129,170)(H,130,177)(H,131,169)(H,132,171)(H,133,167)(H,134,174)(H,135,175)(H,136,172)(H,137,173)(H,138,164)(H,139,168)(H,151,152)(H,153,154)(H,155,156)(H,157,158)(H,159,160)(H,161,162)(H,180,181)(H4,117,118,120)/t57-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,94-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072537
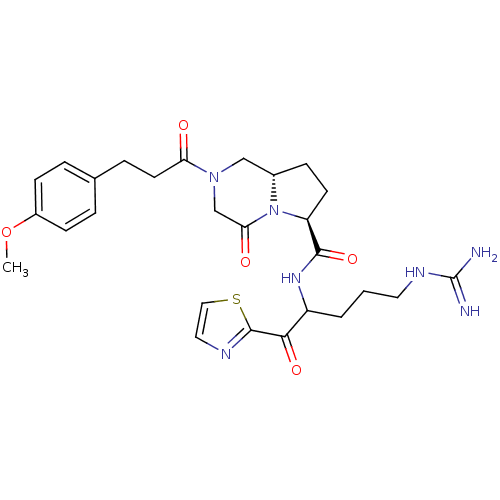
((6S,8aS)-2-[3-(4-Methoxy-phenyl)-propionyl]-4-oxo-...)Show SMILES COc1ccc(CCC(=O)N2C[C@@H]3CC[C@H](N3C(=O)C2)C(=O)NC(CCCNC(N)=N)C(=O)c2nccs2)cc1 Show InChI InChI=1S/C27H35N7O5S/c1-39-19-8-4-17(5-9-19)6-11-22(35)33-15-18-7-10-21(34(18)23(36)16-33)25(38)32-20(3-2-12-31-27(28)29)24(37)26-30-13-14-40-26/h4-5,8-9,13-14,18,20-21H,2-3,6-7,10-12,15-16H2,1H3,(H,32,38)(H4,28,29,31)/t18-,20?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072534
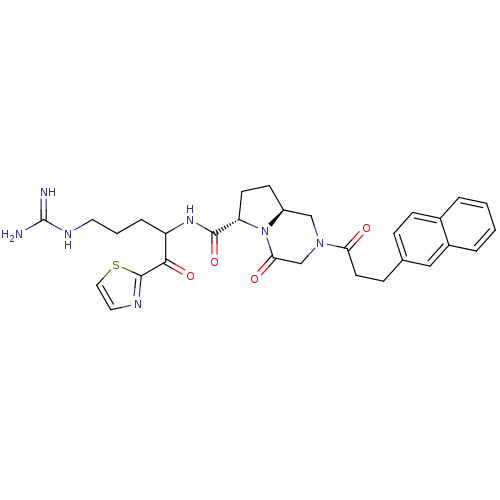
((6S,8aS)-2-(3-Naphthalen-2-yl-propionyl)-4-oxo-oct...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccc2ccccc2c1)C(=O)c1nccs1 Show InChI InChI=1S/C30H35N7O4S/c31-30(32)34-13-3-6-23(27(40)29-33-14-15-42-29)35-28(41)24-11-10-22-17-36(18-26(39)37(22)24)25(38)12-8-19-7-9-20-4-1-2-5-21(20)16-19/h1-2,4-5,7,9,14-16,22-24H,3,6,8,10-13,17-18H2,(H,35,41)(H4,31,32,34)/t22-,23?,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072527
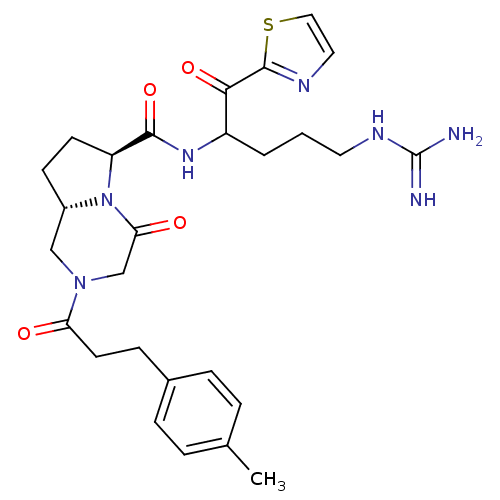
((6S,8aS)-4-Oxo-2-(3-p-tolyl-propionyl)-octahydro-p...)Show SMILES Cc1ccc(CCC(=O)N2C[C@@H]3CC[C@H](N3C(=O)C2)C(=O)NC(CCCNC(N)=N)C(=O)c2nccs2)cc1 Show InChI InChI=1S/C27H35N7O4S/c1-17-4-6-18(7-5-17)8-11-22(35)33-15-19-9-10-21(34(19)23(36)16-33)25(38)32-20(3-2-12-31-27(28)29)24(37)26-30-13-14-39-26/h4-7,13-14,19-21H,2-3,8-12,15-16H2,1H3,(H,32,38)(H4,28,29,31)/t19-,20?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072524
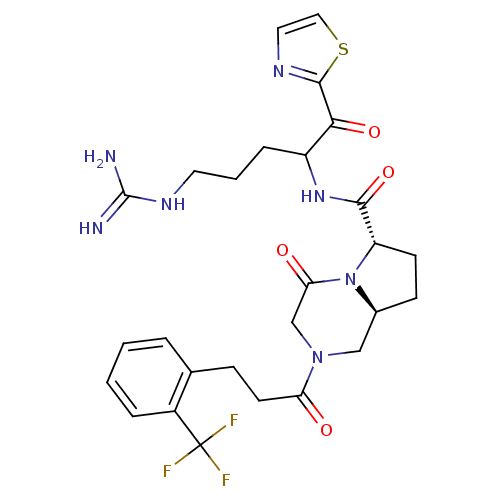
((6S,8aS)-4-Oxo-2-[3-(2-trifluoromethyl-phenyl)-pro...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccccc1C(F)(F)F)C(=O)c1nccs1 Show InChI InChI=1S/C27H32F3N7O4S/c28-27(29,30)18-5-2-1-4-16(18)7-10-21(38)36-14-17-8-9-20(37(17)22(39)15-36)24(41)35-19(6-3-11-34-26(31)32)23(40)25-33-12-13-42-25/h1-2,4-5,12-13,17,19-20H,3,6-11,14-15H2,(H,35,41)(H4,31,32,34)/t17-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004745
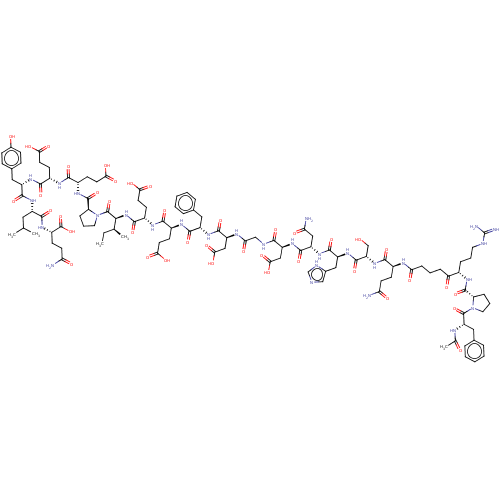
(CHEMBL2370450 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)CCCC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O |wU:75.79,57.62,95.104,8.13,153.157,135.139,wD:131.136,4.4,106.107,65.73,49.54,26.34,17.22,37.38,165.170,113.124,81.88,144.148,173.178,2.2,(30.53,-12.45,;32.01,-12.05,;32.41,-10.56,;31.32,-9.47,;33.9,-10.16,;34.29,-8.67,;33.2,-7.58,;31.72,-7.98,;33.6,-6.1,;35.09,-5.7,;35.48,-4.21,;36.97,-3.81,;37.37,-2.32,;38.06,-4.89,;32.51,-5.01,;32.91,-3.52,;34.39,-3.12,;31.82,-2.43,;30.33,-2.83,;29.93,-4.32,;28.44,-4.72,;27.35,-3.63,;28.05,-6.21,;32.21,-.94,;31.12,.14,;29.63,-.26,;31.52,1.63,;30.43,2.72,;30.82,4.21,;32.31,4.61,;32.71,6.09,;31.62,7.18,;30.13,6.78,;29.73,5.29,;33,2.03,;34.1,.94,;33.7,-.54,;35.58,1.34,;35.98,2.83,;34.89,3.92,;33.4,3.52,;35.28,5.41,;36.67,.26,;38.16,.66,;38.56,2.15,;39.25,-.43,;40.74,-.03,;41.83,-1.12,;41.43,-2.6,;43.31,-.72,;43.71,.77,;45.2,1.17,;45.59,2.66,;46.72,.93,;44.4,-1.8,;45.89,-1.4,;46.29,.09,;46.98,-2.49,;46.59,-3.98,;47.68,-5.07,;49.11,-5.62,;47.28,-6.55,;48.47,-2.09,;49.56,-3.18,;49.16,-4.67,;51.05,-2.78,;51.44,-1.29,;52.93,-.89,;54.13,-1.86,;55.42,-1.01,;55.02,.47,;53.48,.55,;52.14,-3.86,;53.62,-3.46,;54.82,-2.49,;54.71,-4.55,;54.32,-6.04,;52.83,-6.44,;56.2,-4.15,;57.29,-5.24,;56.9,-6.73,;58.78,-4.84,;59.18,-3.35,;58.08,-2.26,;58.48,-.77,;59.97,-.37,;57.39,.31,;59.87,-5.92,;61.36,-5.52,;61.75,-4.04,;62.45,-6.61,;63.93,-6.21,;65.02,-7.3,;66.51,-6.9,;66.91,-5.41,;67.6,-7.98,;67.21,-9.47,;68.3,-10.56,;67.9,-12.05,;68.99,-13.14,;68.59,-14.62,;67.11,-15.02,;69.68,-15.71,;69.09,-7.58,;69.49,-6.1,;68.39,-5.01,;70.97,-5.7,;71.52,-4.26,;73.06,-4.34,;73.46,-5.82,;72.17,-6.66,;72.09,-8.2,;70.72,-8.9,;73.38,-9.04,;74.76,-8.34,;76.05,-9.17,;75.97,-10.71,;77.26,-11.55,;78.63,-10.85,;78.71,-9.31,;77.42,-8.47,;73.31,-10.58,;74.6,-11.41,;74.52,-12.95,;76.12,-11.65,;34.99,-11.25,;36.47,-10.85,;34.59,-12.74,;33.15,-13.29,;33.24,-14.83,;34.72,-15.22,;35.56,-13.93,;37.1,-13.85,;37.8,-12.47,;37.94,-15.14,;39.48,-15.06,;40.17,-13.68,;39.33,-12.39,;40.03,-11.02,;39.19,-9.73,;41.57,-10.94,;40.32,-16.35,;39.62,-17.72,;41.86,-16.26,;42.7,-17.55,;42,-18.93,;40.46,-19.01,;39.76,-20.38,;40.6,-21.67,;38.23,-20.47,;44.23,-17.47,;44.93,-16.1,;45.07,-18.76,;46.61,-18.68,;47.31,-17.31,;48.85,-17.22,;49.69,-18.51,;51.23,-18.43,;51.92,-17.06,;53.46,-16.97,;51.08,-15.77,;49.54,-15.85,;47.45,-19.97,;46.76,-21.34,;48.99,-19.89,;49.83,-21.18,;49.13,-22.55,;49.97,-23.84,;49.28,-25.21,;51.51,-23.76,;51.37,-21.09,;52.07,-19.72,;52.21,-22.38,;53.75,-22.3,;54.59,-23.59,;56.13,-23.51,;56.97,-24.8,;56.27,-26.17,;58.5,-24.72,;54.44,-20.93,;53.6,-19.64,;55.98,-20.85,)| Show InChI InChI=1S/C114H160N28O40/c1-6-58(4)95(112(180)142-44-16-23-82(142)110(178)131-69(34-40-91(156)157)99(167)128-68(33-39-90(154)155)100(168)134-74(47-62-26-28-64(145)29-27-62)104(172)133-72(45-57(2)3)102(170)132-71(113(181)182)31-37-85(116)148)140-101(169)70(35-41-92(158)159)129-98(166)67(32-38-89(152)153)130-103(171)73(46-60-17-9-7-10-18-60)135-107(175)78(52-94(162)163)126-88(151)54-122-96(164)77(51-93(160)161)138-106(174)76(50-86(117)149)137-105(173)75(49-63-53-120-56-123-63)136-108(176)80(55-143)139-97(165)66(30-36-84(115)147)125-87(150)25-13-24-83(146)65(21-14-42-121-114(118)119)127-109(177)81-22-15-43-141(81)111(179)79(124-59(5)144)48-61-19-11-8-12-20-61/h7-12,17-20,26-29,53,56-58,65-82,95,143,145H,6,13-16,21-25,30-52,54-55H2,1-5H3,(H2,115,147)(H2,116,148)(H2,117,149)(H,120,123)(H,122,164)(H,124,144)(H,125,150)(H,126,151)(H,127,177)(H,128,167)(H,129,166)(H,130,171)(H,131,178)(H,132,170)(H,133,172)(H,134,168)(H,135,175)(H,136,176)(H,137,173)(H,138,174)(H,139,165)(H,140,169)(H,152,153)(H,154,155)(H,156,157)(H,158,159)(H,160,161)(H,162,163)(H,181,182)(H4,118,119,121)/t58-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,95-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072532

((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C26H33N7O4S/c27-26(28)30-12-4-7-19(23(36)25-29-13-14-38-25)31-24(37)20-10-9-18-15-32(16-22(35)33(18)20)21(34)11-8-17-5-2-1-3-6-17/h1-3,5-6,13-14,18-20H,4,7-12,15-16H2,(H,31,37)(H4,27,28,30)/t18-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072520
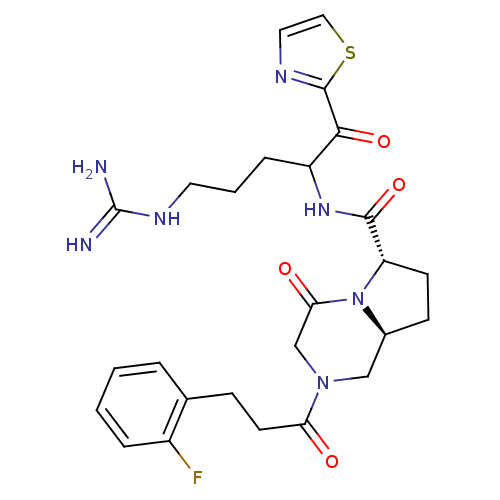
((6S,8aS)-2-[3-(2-Fluoro-phenyl)-propionyl]-4-oxo-o...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccccc1F)C(=O)c1nccs1 Show InChI InChI=1S/C26H32FN7O4S/c27-18-5-2-1-4-16(18)7-10-21(35)33-14-17-8-9-20(34(17)22(36)15-33)24(38)32-19(6-3-11-31-26(28)29)23(37)25-30-12-13-39-25/h1-2,4-5,12-13,17,19-20H,3,6-11,14-15H2,(H,32,38)(H4,28,29,31)/t17-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072533
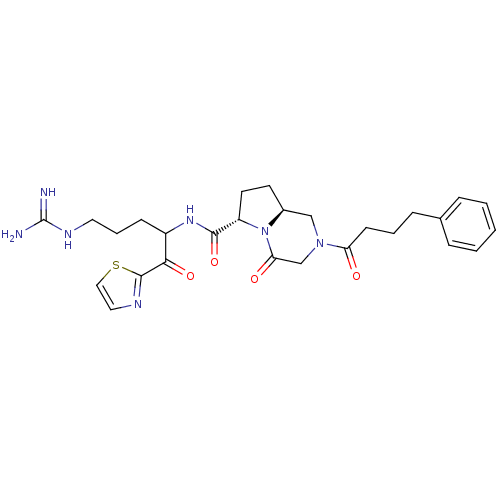
((6S,8aS)-4-Oxo-2-(4-phenyl-butyryl)-octahydro-pyrr...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C27H35N7O4S/c28-27(29)31-13-5-9-20(24(37)26-30-14-15-39-26)32-25(38)21-12-11-19-16-33(17-23(36)34(19)21)22(35)10-4-8-18-6-2-1-3-7-18/h1-3,6-7,14-15,19-21H,4-5,8-13,16-17H2,(H,32,38)(H4,28,29,31)/t19-,20?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50076511
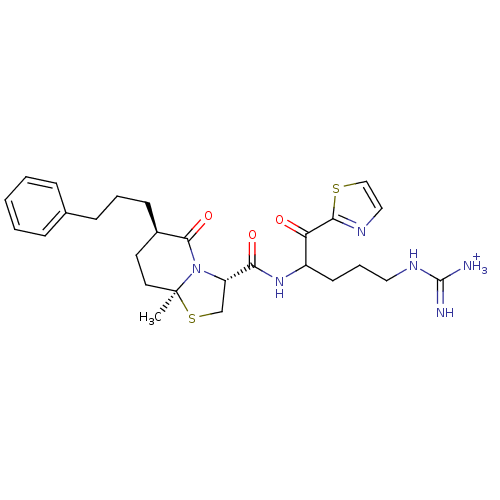
((3R,6R,8aS)-8a-Methyl-5-oxo-6-(3-phenyl-propyl)-he...)Show SMILES C[C@]12CC[C@@H](CCCc3ccccc3)C(=O)N1[C@@H](CS2)C(=O)NC(CCCNC([NH3+])=N)C(=O)c1nccs1 Show InChI InChI=1S/C27H36N6O3S2/c1-27-13-12-19(10-5-9-18-7-3-2-4-8-18)25(36)33(27)21(17-38-27)23(35)32-20(11-6-14-31-26(28)29)22(34)24-30-15-16-37-24/h2-4,7-8,15-16,19-21H,5-6,9-14,17H2,1H3,(H,32,35)(H4,28,29,31)/p+1/t19-,20?,21+,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human thrombin.( Fast moving component on HPLC) |
Bioorg Med Chem Lett 9: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2V98780 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027508
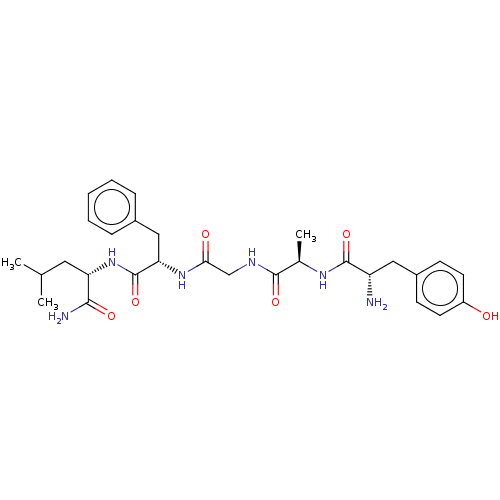
(Acetate1-(6-benzyl-3-isobutyl-2,5,8,11-tetraoxo-1,...)Show SMILES CC(O)=O.CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C29H40N6O6.C2H4O2/c1-17(2)13-23(26(31)38)35-29(41)24(15-19-7-5-4-6-8-19)34-25(37)16-32-27(39)18(3)33-28(40)22(30)14-20-9-11-21(36)12-10-20;1-2(3)4/h4-12,17-18,22-24,36H,13-16,30H2,1-3H3,(H2,31,38)(H,32,39)(H,33,40)(H,34,37)(H,35,41);1H3,(H,3,4)/t18-,22+,23+,24+;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027512

(Acetate1-[1-({[1-(1-carbamoyl-3-methyl-butylcarbam...)Show SMILES CC(O)=O.CCCC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C32H46N6O6.C2H4O2/c1-4-5-11-25(37-30(42)24(33)17-22-12-14-23(39)15-13-22)31(43)35-19-28(40)36-27(18-21-9-7-6-8-10-21)32(44)38-26(29(34)41)16-20(2)3;1-2(3)4/h6-10,12-15,20,24-27,39H,4-5,11,16-19,33H2,1-3H3,(H2,34,41)(H,35,43)(H,36,40)(H,37,42)(H,38,44);1H3,(H,3,4)/t24-,25+,26-,27-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027508

(Acetate1-(6-benzyl-3-isobutyl-2,5,8,11-tetraoxo-1,...)Show SMILES CC(O)=O.CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C29H40N6O6.C2H4O2/c1-17(2)13-23(26(31)38)35-29(41)24(15-19-7-5-4-6-8-19)34-25(37)16-32-27(39)18(3)33-28(40)22(30)14-20-9-11-21(36)12-10-20;1-2(3)4/h4-12,17-18,22-24,36H,13-16,30H2,1-3H3,(H2,31,38)(H,32,39)(H,33,40)(H,34,37)(H,35,41);1H3,(H,3,4)/t18-,22+,23+,24+;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072530
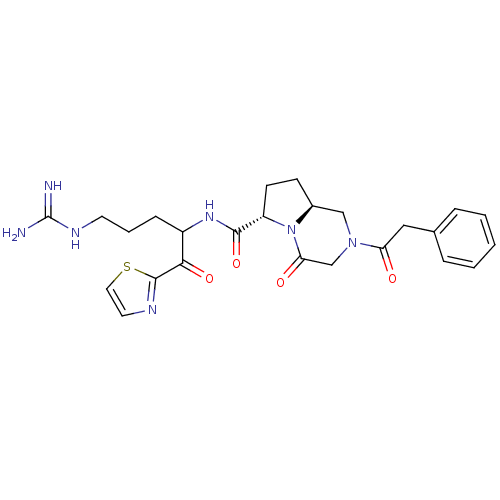
((6S,8aS)-4-Oxo-2-phenylacetyl-octahydro-pyrrolo[1,...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)Cc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C25H31N7O4S/c26-25(27)29-10-4-7-18(22(35)24-28-11-12-37-24)30-23(36)19-9-8-17-14-31(15-21(34)32(17)19)20(33)13-16-5-2-1-3-6-16/h1-3,5-6,11-12,17-19H,4,7-10,13-15H2,(H,30,36)(H4,26,27,29)/t17-,18?,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004743

(Ac-(D)Phe-Pro-Arg.Pro.Gln.Ser-H~s.Asn-AspGly-Asp-P...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C92H132N24O28/c1-7-49(4)75(90(142)115-35-17-25-65(115)84(136)109-62(91(143)144)37-48(2)3)111-85(137)66-26-15-33-113(66)86(138)55(29-31-72(122)123)104-78(130)56(38-51-19-10-8-11-20-51)105-81(133)60(43-74(126)127)102-71(121)45-99-76(128)59(42-73(124)125)108-80(132)58(41-70(94)120)107-79(131)57(40-53-44-97-47-100-53)106-82(134)63(46-117)110-77(129)54(28-30-69(93)119)103-83(135)64-24-16-34-114(64)89(141)67(23-14-32-98-92(95)96)112(6)88(140)68-27-18-36-116(68)87(139)61(101-50(5)118)39-52-21-12-9-13-22-52/h8-13,19-22,44,47-49,54-68,75,117H,7,14-18,23-43,45-46H2,1-6H3,(H2,93,119)(H2,94,120)(H,97,100)(H,99,128)(H,101,118)(H,102,121)(H,103,135)(H,104,130)(H,105,133)(H,106,134)(H,107,131)(H,108,132)(H,109,136)(H,110,129)(H,111,137)(H,122,123)(H,124,125)(H,126,127)(H,143,144)(H4,95,96,98)/t49-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,75-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072519

((6S,8aS)-2-[(S)-2-Amino-3-(4-fluoro-phenyl)-propio...)Show SMILES N[C@@H](Cc1ccc(F)cc1)C(=O)N1C[C@@H]2CC[C@H](N2C(=O)C1)C(=O)NC(CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C26H33FN8O4S/c27-16-5-3-15(4-6-16)12-18(28)25(39)34-13-17-7-8-20(35(17)21(36)14-34)23(38)33-19(2-1-9-32-26(29)30)22(37)24-31-10-11-40-24/h3-6,10-11,17-20H,1-2,7-9,12-14,28H2,(H,33,38)(H4,29,30,32)/t17-,18-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072541

((6S,8aS)-4-Oxo-2-(3-pyridin-2-yl-propionyl)-octahy...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccccn1)C(=O)c1nccs1 Show InChI InChI=1S/C25H32N8O4S/c26-25(27)30-11-3-5-18(22(36)24-29-12-13-38-24)31-23(37)19-8-7-17-14-32(15-21(35)33(17)19)20(34)9-6-16-4-1-2-10-28-16/h1-2,4,10,12-13,17-19H,3,5-9,11,14-15H2,(H,31,37)(H4,26,27,30)/t17-,18?,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027506
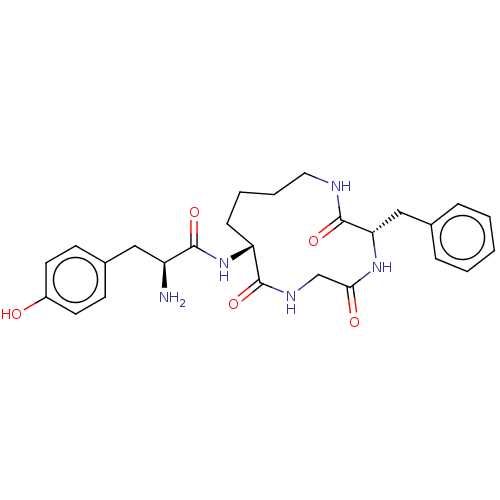
(Acetate1-(3-benzyl-2,5,8-trioxo-1,4,7triaza-cyclot...)Show SMILES CC(O)=O.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C26H33N5O5.C2H4O2/c27-20(14-18-9-11-19(32)12-10-18)24(34)31-21-8-4-5-13-28-26(36)22(15-17-6-2-1-3-7-17)30-23(33)16-29-25(21)35;1-2(3)4/h1-3,6-7,9-12,20-22,32H,4-5,8,13-16,27H2,(H,28,36)(H,29,35)(H,30,33)(H,31,34);1H3,(H,3,4)/t20-,21+,22-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027511
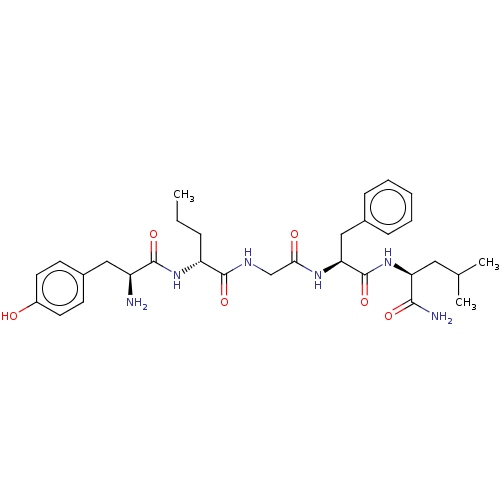
(Acetate1-[1-({[1-(1-carbamoyl-3-methyl-butylcarbam...)Show SMILES CC(O)=O.CCC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C31H44N6O6.C2H4O2/c1-4-8-24(36-29(41)23(32)16-21-11-13-22(38)14-12-21)30(42)34-18-27(39)35-26(17-20-9-6-5-7-10-20)31(43)37-25(28(33)40)15-19(2)3;1-2(3)4/h5-7,9-14,19,23-26,38H,4,8,15-18,32H2,1-3H3,(H2,33,40)(H,34,42)(H,35,39)(H,36,41)(H,37,43);1H3,(H,3,4)/t23-,24+,25-,26-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H][D-Ala2,D-Leu5]enkephalin binding to Opioid receptor delta 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004742
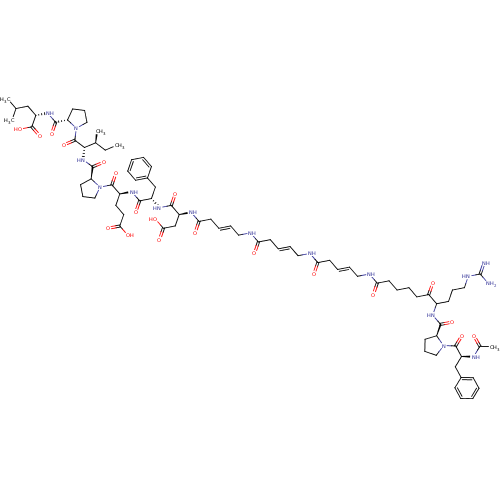
(CHEMBL385670 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)C\C=C\CNC(=O)C\C=C\CNC(=O)C\C=C\CNC(=O)CCCCC(=O)C(CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C82H118N16O20/c1-6-52(4)72(80(116)98-46-24-31-63(98)76(112)94-61(81(117)118)47-51(2)3)95-77(113)64-32-23-44-96(64)78(114)57(38-39-70(105)106)92-73(109)58(48-54-25-9-7-10-26-54)93-74(110)59(50-71(107)108)90-69(104)37-17-20-42-87-68(103)36-16-19-41-86-67(102)35-15-18-40-85-66(101)34-14-13-33-65(100)56(29-21-43-88-82(83)84)91-75(111)62-30-22-45-97(62)79(115)60(89-53(5)99)49-55-27-11-8-12-28-55/h7-12,15-20,25-28,51-52,56-64,72H,6,13-14,21-24,29-50H2,1-5H3,(H,85,101)(H,86,102)(H,87,103)(H,89,99)(H,90,104)(H,91,111)(H,92,109)(H,93,110)(H,94,112)(H,95,113)(H,105,106)(H,107,108)(H,117,118)(H4,83,84,88)/b18-15+,19-16+,20-17+/t52-,56?,57-,58-,59-,60-,61-,62-,63-,64-,72-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027511

(Acetate1-[1-({[1-(1-carbamoyl-3-methyl-butylcarbam...)Show SMILES CC(O)=O.CCC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C31H44N6O6.C2H4O2/c1-4-8-24(36-29(41)23(32)16-21-11-13-22(38)14-12-21)30(42)34-18-27(39)35-26(17-20-9-6-5-7-10-20)31(43)37-25(28(33)40)15-19(2)3;1-2(3)4/h5-7,9-14,19,23-26,38H,4,8,15-18,32H2,1-3H3,(H2,33,40)(H,34,42)(H,35,39)(H,36,41)(H,37,43);1H3,(H,3,4)/t23-,24+,25-,26-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004741
(CHEMBL427978 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)C\C=C\CNC(=O)C\C=C\CNC(=O)CCCCC(=O)C(CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C77H111N15O19/c1-6-48(4)67(75(109)92-42-22-29-59(92)71(105)88-57(76(110)111)43-47(2)3)89-72(106)60-30-21-40-90(60)73(107)53(35-36-65(98)99)86-68(102)54(44-50-23-9-7-10-24-50)87-69(103)55(46-66(100)101)84-64(97)34-16-18-38-81-63(96)33-15-17-37-80-62(95)32-14-13-31-61(94)52(27-19-39-82-77(78)79)85-70(104)58-28-20-41-91(58)74(108)56(83-49(5)93)45-51-25-11-8-12-26-51/h7-12,15-18,23-26,47-48,52-60,67H,6,13-14,19-22,27-46H2,1-5H3,(H,80,95)(H,81,96)(H,83,93)(H,84,97)(H,85,104)(H,86,102)(H,87,103)(H,88,105)(H,89,106)(H,98,99)(H,100,101)(H,110,111)(H4,78,79,82)/b17-15+,18-16+/t48-,52?,53-,54-,55-,56-,57-,58-,59-,60-,67-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004738
(CHEMBL2370451 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)CC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O |wU:129.134,104.105,75.79,57.62,93.102,8.13,163.168,111.122,142.146,171.176,wD:4.4,65.73,49.54,26.34,17.22,37.38,151.155,81.88,133.137,2.2,(44.24,-11.55,;45.73,-11.18,;46.16,-9.7,;45.09,-8.59,;47.66,-9.33,;48.08,-7.85,;47.02,-6.74,;45.52,-7.11,;47.44,-5.26,;48.94,-4.89,;49.36,-3.41,;50.86,-3.04,;51.29,-1.56,;51.93,-4.15,;46.37,-4.15,;46.8,-2.67,;48.3,-2.3,;45.73,-1.56,;44.24,-1.93,;43.81,-3.41,;42.32,-3.78,;41.25,-2.67,;41.89,-5.26,;46.16,-.08,;45.09,1.03,;43.6,.66,;45.52,2.51,;44.45,3.62,;44.88,5.1,;46.37,5.47,;46.8,6.94,;45.73,8.05,;44.24,7.68,;43.81,6.2,;47.02,2.88,;48.08,1.77,;47.66,.29,;49.58,2.14,;50.01,3.62,;48.94,4.73,;47.44,4.36,;49.36,6.2,;50.65,1.03,;52.14,1.4,;52.57,2.88,;53.21,.29,;54.7,.66,;55.77,-.45,;55.34,-1.93,;57.27,-.08,;57.69,1.4,;59.19,1.77,;59.62,3.25,;60.7,1.5,;58.33,-1.19,;59.83,-.82,;60.26,.66,;60.9,-1.93,;60.47,-3.41,;61.54,-4.52,;61.11,-6,;62.96,-5.1,;62.39,-1.56,;63.46,-2.67,;63.03,-4.15,;64.95,-2.3,;65.38,-.82,;66.88,-.45,;68.05,-1.44,;69.36,-.63,;68.99,.86,;67.46,.97,;66.02,-3.41,;67.52,-3.04,;68.73,-2.1,;68.58,-4.15,;68.16,-5.63,;66.66,-6,;70.08,-3.78,;71.15,-4.89,;70.72,-6.37,;72.64,-4.52,;73.07,-3.04,;72,-1.93,;72.43,-.45,;71.36,.66,;73.92,-.08,;73.71,-5.63,;75.21,-5.26,;75.63,-3.78,;76.27,-6.37,;77.77,-6,;78.2,-4.52,;78.84,-7.11,;78.41,-8.59,;79.48,-9.7,;79.05,-11.18,;80.12,-12.29,;79.69,-13.77,;78.2,-14.14,;80.76,-14.88,;80.33,-6.74,;80.76,-5.26,;79.69,-4.15,;82.25,-4.89,;83.43,-5.88,;84.74,-5.07,;84.37,-3.58,;82.83,-3.47,;82.02,-2.16,;80.48,-2.21,;82.74,-.8,;84.28,-.75,;85.01,.61,;86.55,.66,;87.27,2.02,;86.46,3.33,;84.92,3.28,;84.19,1.92,;81.93,.51,;82.66,1.87,;83.87,2.81,;81.84,3.17,;48.72,-10.44,;48.3,-11.92,;50.22,-10.07,;50.8,-8.64,;52.33,-8.75,;52.7,-10.25,;51.4,-11.06,;51.29,-12.6,;49.9,-13.27,;52.56,-13.46,;52.45,-15,;51.07,-15.67,;49.79,-14.81,;48.41,-15.48,;47.13,-14.62,;48.3,-17.02,;53.73,-15.86,;55.11,-15.19,;53.62,-17.4,;54.89,-18.26,;56.28,-17.59,;56.39,-16.05,;57.77,-15.38,;59.05,-16.24,;57.88,-13.84,;54.78,-19.8,;53.4,-20.47,;56.06,-20.66,;55.95,-22.2,;54.56,-22.87,;54.45,-24.4,;53.07,-25.08,;52.96,-26.61,;54.23,-27.48,;54.12,-29.01,;55.62,-26.8,;55.73,-25.27,;57.22,-23.06,;58.61,-22.39,;57.11,-24.6,;58.39,-25.46,;59.77,-24.79,;61.05,-25.65,;62.43,-24.98,;60.94,-27.19,;58.28,-27,;56.89,-27.67,;59.55,-27.86,;59.44,-29.39,;60.72,-30.26,;60.61,-31.79,;61.88,-32.66,;63.27,-31.99,;61.77,-34.19,;58.06,-30.07,;56.78,-29.2,;57.95,-31.6,)| Show InChI InChI=1S/C112H156N28O40/c1-6-56(4)93(110(178)140-41-15-22-80(140)108(176)129-67(31-37-89(154)155)97(165)126-66(30-36-88(152)153)98(166)132-72(44-60-23-25-62(143)26-24-60)102(170)131-70(42-55(2)3)100(168)130-69(111(179)180)28-34-83(114)146)138-99(167)68(32-38-90(156)157)127-96(164)65(29-35-87(150)151)128-101(169)71(43-58-16-9-7-10-17-58)133-105(173)76(49-92(160)161)124-86(149)52-120-94(162)75(48-91(158)159)136-104(172)74(47-84(115)147)135-103(171)73(46-61-51-118-54-121-61)134-106(174)78(53-141)137-95(163)64(27-33-82(113)145)123-85(148)50-81(144)63(20-13-39-119-112(116)117)125-107(175)79-21-14-40-139(79)109(177)77(122-57(5)142)45-59-18-11-8-12-19-59/h7-12,16-19,23-26,51,54-56,63-80,93,141,143H,6,13-15,20-22,27-50,52-53H2,1-5H3,(H2,113,145)(H2,114,146)(H2,115,147)(H,118,121)(H,120,162)(H,122,142)(H,123,148)(H,124,149)(H,125,175)(H,126,165)(H,127,164)(H,128,169)(H,129,176)(H,130,168)(H,131,170)(H,132,166)(H,133,173)(H,134,174)(H,135,171)(H,136,172)(H,137,163)(H,138,167)(H,150,151)(H,152,153)(H,154,155)(H,156,157)(H,158,159)(H,160,161)(H,179,180)(H4,116,117,119)/t56-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,93-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027505

(Acetate1-[1-({[1-(1-carbamoyl-3-methyl-butylcarbam...)Show SMILES CC(O)=O.CC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C30H42N6O6.C2H4O2/c1-4-23(35-28(40)22(31)15-20-10-12-21(37)13-11-20)29(41)33-17-26(38)34-25(16-19-8-6-5-7-9-19)30(42)36-24(27(32)39)14-18(2)3;1-2(3)4/h5-13,18,22-25,37H,4,14-17,31H2,1-3H3,(H2,32,39)(H,33,41)(H,34,38)(H,35,40)(H,36,42);1H3,(H,3,4)/t22-,23+,24-,25-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072529
((6S,8aS)-2-((S)-2-Hydroxy-3-phenyl-propionyl)-4-ox...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)[C@@H](O)Cc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C26H33N7O5S/c27-26(28)30-10-4-7-18(22(36)24-29-11-12-39-24)31-23(37)19-9-8-17-14-32(15-21(35)33(17)19)25(38)20(34)13-16-5-2-1-3-6-16/h1-3,5-6,11-12,17-20,34H,4,7-10,13-15H2,(H,31,37)(H4,27,28,30)/t17-,18?,19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027512

(Acetate1-[1-({[1-(1-carbamoyl-3-methyl-butylcarbam...)Show SMILES CC(O)=O.CCCC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C32H46N6O6.C2H4O2/c1-4-5-11-25(37-30(42)24(33)17-22-12-14-23(39)15-13-22)31(43)35-19-28(40)36-27(18-21-9-7-6-8-10-21)32(44)38-26(29(34)41)16-20(2)3;1-2(3)4/h6-10,12-15,20,24-27,39H,4-5,11,16-19,33H2,1-3H3,(H2,34,41)(H,35,43)(H,36,40)(H,37,42)(H,38,44);1H3,(H,3,4)/t24-,25+,26-,27-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H][D-Ala2,D-Leu5]enkephalin binding to Opioid receptor delta 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50452138
(CHEMBL2372196)Show SMILES CC(O)=O.CC(C)C[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](CCCCNC1=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C32H44N6O6.C2H4O2/c1-20(2)16-26-31(43)34-15-7-6-10-25(37-29(41)24(33)17-22-11-13-23(39)14-12-22)30(42)35-19-28(40)36-27(32(44)38-26)18-21-8-4-3-5-9-21;1-2(3)4/h3-5,8-9,11-14,20,24-27,39H,6-7,10,15-19,33H2,1-2H3,(H,34,43)(H,35,42)(H,36,40)(H,37,41)(H,38,44);1H3,(H,3,4)/t24-,25+,26+,27-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072535
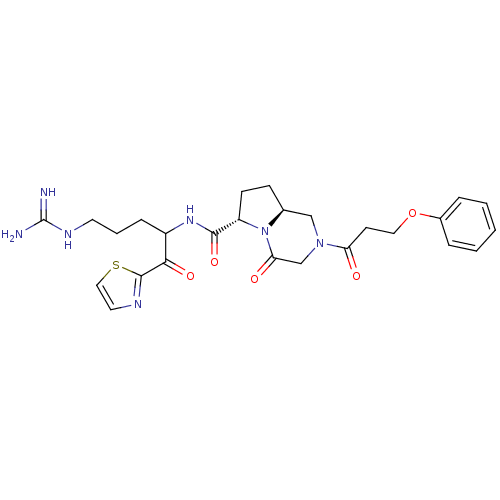
((6S,8aS)-4-Oxo-2-(3-phenoxy-propionyl)-octahydro-p...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCOc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C26H33N7O5S/c27-26(28)30-11-4-7-19(23(36)25-29-12-14-39-25)31-24(37)20-9-8-17-15-32(16-22(35)33(17)20)21(34)10-13-38-18-5-2-1-3-6-18/h1-3,5-6,12,14,17,19-20H,4,7-11,13,15-16H2,(H,31,37)(H4,27,28,30)/t17-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027505

(Acetate1-[1-({[1-(1-carbamoyl-3-methyl-butylcarbam...)Show SMILES CC(O)=O.CC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C30H42N6O6.C2H4O2/c1-4-23(35-28(40)22(31)15-20-10-12-21(37)13-11-20)29(41)33-17-26(38)34-25(16-19-8-6-5-7-9-19)30(42)36-24(27(32)39)14-18(2)3;1-2(3)4/h5-13,18,22-25,37H,4,14-17,31H2,1-3H3,(H2,32,39)(H,33,41)(H,34,38)(H,35,40)(H,36,42);1H3,(H,3,4)/t22-,23+,24-,25-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50076511

((3R,6R,8aS)-8a-Methyl-5-oxo-6-(3-phenyl-propyl)-he...)Show SMILES C[C@]12CC[C@@H](CCCc3ccccc3)C(=O)N1[C@@H](CS2)C(=O)NC(CCCNC([NH3+])=N)C(=O)c1nccs1 Show InChI InChI=1S/C27H36N6O3S2/c1-27-13-12-19(10-5-9-18-7-3-2-4-8-18)25(36)33(27)21(17-38-27)23(35)32-20(11-6-14-31-26(28)29)22(34)24-30-15-16-37-24/h2-4,7-8,15-16,19-21H,5-6,9-14,17H2,1H3,(H,32,35)(H4,28,29,31)/p+1/t19-,20?,21+,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human thrombin.(Slow moving component on HPLC) |
Bioorg Med Chem Lett 9: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2V98780 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50076509
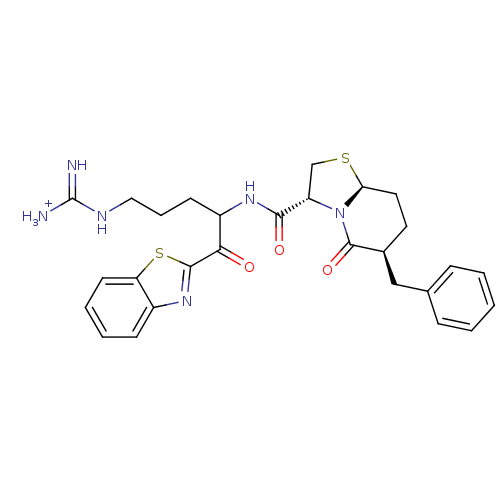
((3R,6S,8aS)-6-Benzyl-5-oxo-hexahydro-thiazolo[3,2-...)Show SMILES [NH3+]C(=N)NCCCC(NC(=O)[C@@H]1CS[C@H]2CC[C@@H](Cc3ccccc3)C(=O)N12)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C28H32N6O3S2/c29-28(30)31-14-6-10-20(24(35)26-33-19-9-4-5-11-22(19)39-26)32-25(36)21-16-38-23-13-12-18(27(37)34(21)23)15-17-7-2-1-3-8-17/h1-5,7-9,11,18,20-21,23H,6,10,12-16H2,(H,32,36)(H4,29,30,31)/p+1/t18-,20?,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human thrombin. |
Bioorg Med Chem Lett 9: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2V98780 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50076507
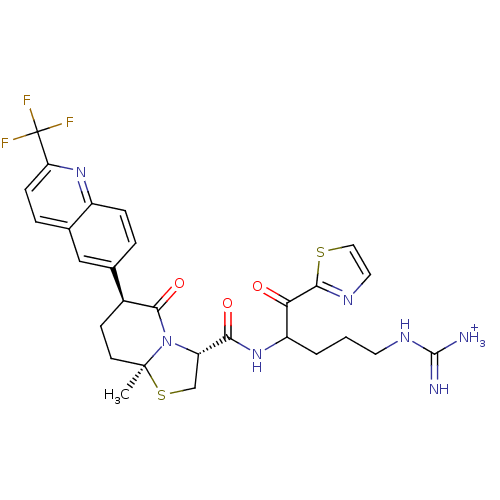
((3R,6S,8aS)-8a-Methyl-5-oxo-6-(2-trifluoromethyl-q...)Show SMILES C[C@]12CC[C@H](C(=O)N1[C@@H](CS2)C(=O)NC(CCCNC([NH3+])=N)C(=O)c1nccs1)c1ccc2nc(ccc2c1)C(F)(F)F Show InChI InChI=1S/C28H30F3N7O3S2/c1-27-9-8-17(15-4-6-18-16(13-15)5-7-21(36-18)28(29,30)31)25(41)38(27)20(14-43-27)23(40)37-19(3-2-10-35-26(32)33)22(39)24-34-11-12-42-24/h4-7,11-13,17,19-20H,2-3,8-10,14H2,1H3,(H,37,40)(H4,32,33,35)/p+1/t17-,19?,20-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human thrombin. |
Bioorg Med Chem Lett 9: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2V98780 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027510
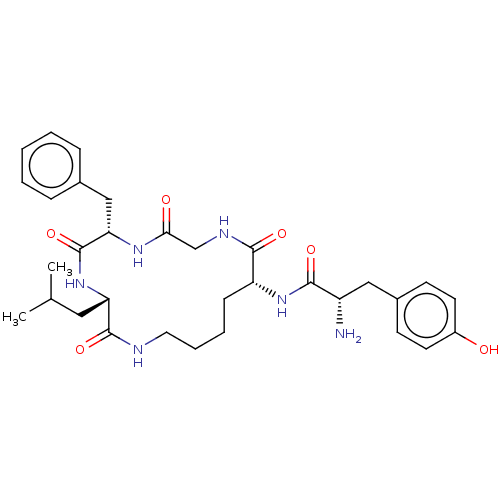
(Acetate1-(6-benzyl-3-isobutyl-2,5,8,11-tetraoxo-1,...)Show SMILES CC(O)=O.CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](CCCCNC1=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C32H44N6O6.C2H4O2/c1-20(2)16-26-31(43)34-15-7-6-10-25(37-29(41)24(33)17-22-11-13-23(39)14-12-22)30(42)35-19-28(40)36-27(32(44)38-26)18-21-8-4-3-5-9-21;1-2(3)4/h3-5,8-9,11-14,20,24-27,39H,6-7,10,15-19,33H2,1-2H3,(H,34,43)(H,35,42)(H,36,40)(H,37,41)(H,38,44);1H3,(H,3,4)/t24-,25+,26-,27-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027503
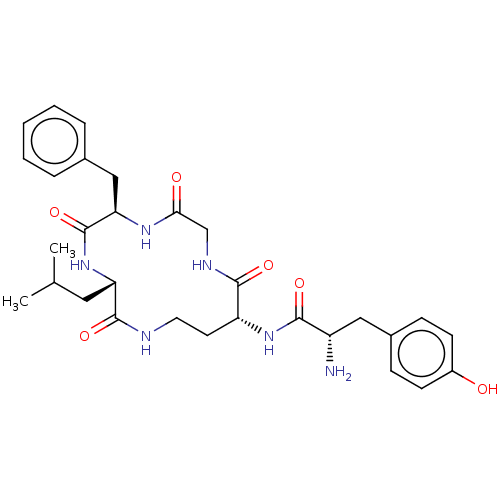
(Acetate1-(6-benzyl-3-isobutyl-2,5,8,11-tetraoxo-1,...)Show SMILES CC(O)=O.CC(C)C[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](CCNC1=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C30H40N6O6.C2H4O2/c1-18(2)14-24-29(41)32-13-12-23(35-27(39)22(31)15-20-8-10-21(37)11-9-20)28(40)33-17-26(38)34-25(30(42)36-24)16-19-6-4-3-5-7-19;1-2(3)4/h3-11,18,22-25,37H,12-17,31H2,1-2H3,(H,32,41)(H,33,40)(H,34,38)(H,35,39)(H,36,42);1H3,(H,3,4)/t22-,23+,24-,25+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50027510

(Acetate1-(6-benzyl-3-isobutyl-2,5,8,11-tetraoxo-1,...)Show SMILES CC(O)=O.CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](CCCCNC1=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C32H44N6O6.C2H4O2/c1-20(2)16-26-31(43)34-15-7-6-10-25(37-29(41)24(33)17-22-11-13-23(39)14-12-22)30(42)35-19-28(40)36-27(32(44)38-26)18-21-8-4-3-5-9-21;1-2(3)4/h3-5,8-9,11-14,20,24-27,39H,6-7,10,15-19,33H2,1-2H3,(H,34,43)(H,35,42)(H,36,40)(H,37,41)(H,38,44);1H3,(H,3,4)/t24-,25+,26-,27-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H][D-Ala2,D-Leu5]enkephalin binding to Opioid receptor delta 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072521
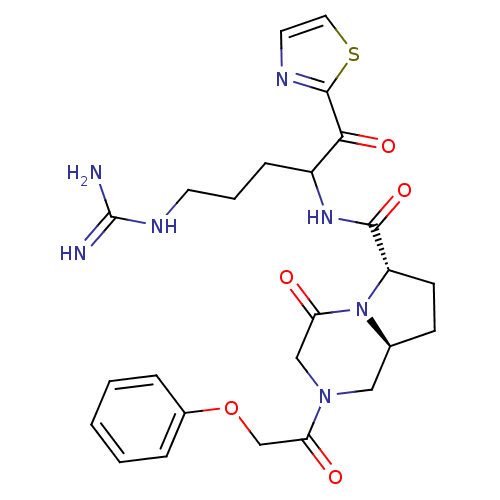
((6S,8aS)-4-Oxo-2-(2-phenoxy-acetyl)-octahydro-pyrr...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)COc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C25H31N7O5S/c26-25(27)29-10-4-7-18(22(35)24-28-11-12-38-24)30-23(36)19-9-8-16-13-31(14-20(33)32(16)19)21(34)15-37-17-5-2-1-3-6-17/h1-3,5-6,11-12,16,18-19H,4,7-10,13-15H2,(H,30,36)(H4,26,27,29)/t16-,18?,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50076506
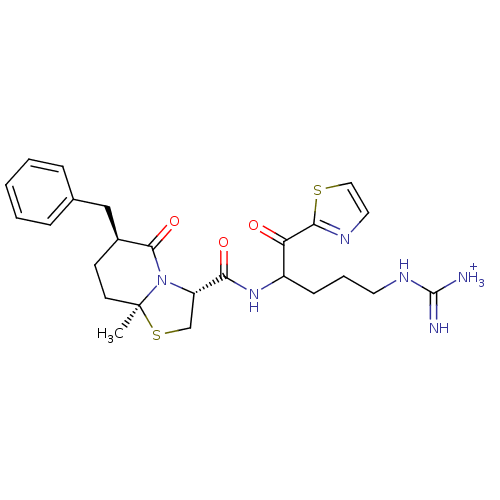
((3R,6S,8aS)-6-Benzyl-8a-methyl-5-oxo-hexahydro-thi...)Show SMILES C[C@]12CC[C@@H](Cc3ccccc3)C(=O)N1[C@@H](CS2)C(=O)NC(CCCNC([NH3+])=N)C(=O)c1nccs1 Show InChI InChI=1S/C25H32N6O3S2/c1-25-10-9-17(14-16-6-3-2-4-7-16)23(34)31(25)19(15-36-25)21(33)30-18(8-5-11-29-24(26)27)20(32)22-28-12-13-35-22/h2-4,6-7,12-13,17-19H,5,8-11,14-15H2,1H3,(H,30,33)(H4,26,27,29)/p+1/t17-,18?,19-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human thrombin. |
Bioorg Med Chem Lett 9: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2V98780 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072538
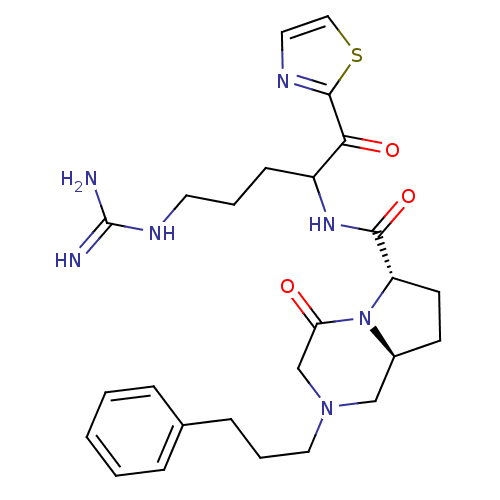
((6S,8aS)-4-Oxo-2-(3-phenyl-propyl)-octahydro-pyrro...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CCCc3ccccc3)CC(=O)N12)C(=O)c1nccs1 Show InChI InChI=1S/C26H35N7O3S/c27-26(28)30-12-4-9-20(23(35)25-29-13-15-37-25)31-24(36)21-11-10-19-16-32(17-22(34)33(19)21)14-5-8-18-6-2-1-3-7-18/h1-3,6-7,13,15,19-21H,4-5,8-12,14,16-17H2,(H,31,36)(H4,27,28,30)/t19-,20?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072526
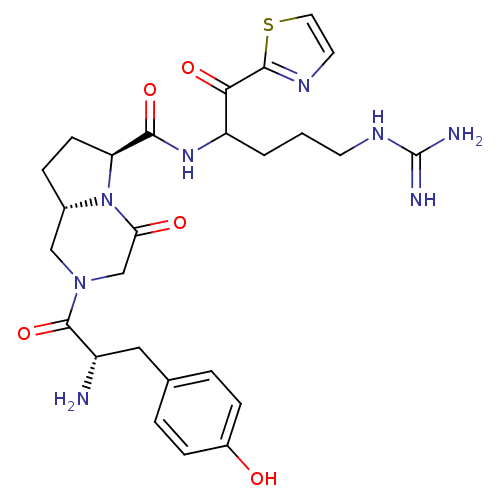
((6S,8aS)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propi...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1C[C@@H]2CC[C@H](N2C(=O)C1)C(=O)NC(CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C26H34N8O5S/c27-18(12-15-3-6-17(35)7-4-15)25(39)33-13-16-5-8-20(34(16)21(36)14-33)23(38)32-19(2-1-9-31-26(28)29)22(37)24-30-10-11-40-24/h3-4,6-7,10-11,16,18-20,35H,1-2,5,8-9,12-14,27H2,(H,32,38)(H4,28,29,31)/t16-,18-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072526

((6S,8aS)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propi...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1C[C@@H]2CC[C@H](N2C(=O)C1)C(=O)NC(CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C26H34N8O5S/c27-18(12-15-3-6-17(35)7-4-15)25(39)33-13-16-5-8-20(34(16)21(36)14-33)23(38)32-19(2-1-9-31-26(28)29)22(37)24-30-10-11-40-24/h3-4,6-7,10-11,16,18-20,35H,1-2,5,8-9,12-14,27H2,(H,32,38)(H4,28,29,31)/t16-,18-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50076505
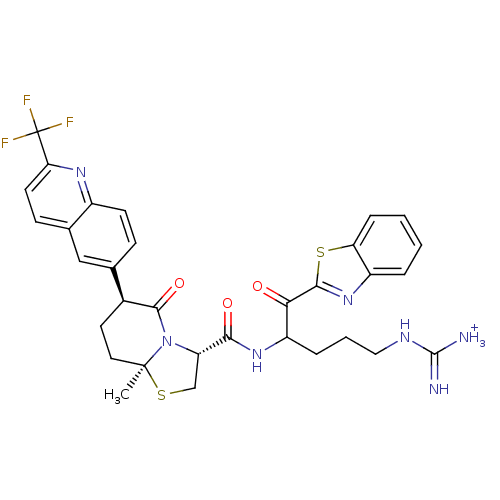
((3R,6S,8aS)-8a-Methyl-5-oxo-6-(2-trifluoromethyl-q...)Show SMILES C[C@]12CC[C@H](C(=O)N1[C@@H](CS2)C(=O)NC(CCCNC([NH3+])=N)C(=O)c1nc2ccccc2s1)c1ccc2nc(ccc2c1)C(F)(F)F Show InChI InChI=1S/C32H32F3N7O3S2/c1-31-13-12-19(17-8-10-20-18(15-17)9-11-25(39-20)32(33,34)35)29(45)42(31)23(16-46-31)27(44)40-22(6-4-14-38-30(36)37)26(43)28-41-21-5-2-3-7-24(21)47-28/h2-3,5,7-11,15,19,22-23H,4,6,12-14,16H2,1H3,(H,40,44)(H4,36,37,38)/p+1/t19-,22?,23-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human thrombin. |
Bioorg Med Chem Lett 9: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2V98780 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50076513
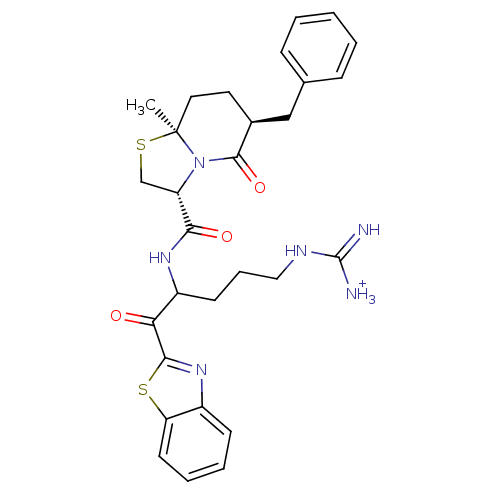
((3R,6S,8aS)-6-Benzyl-8a-methyl-5-oxo-hexahydro-thi...)Show SMILES C[C@]12CC[C@@H](Cc3ccccc3)C(=O)N1[C@@H](CS2)C(=O)NC(CCCNC([NH3+])=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C29H34N6O3S2/c1-29-14-13-19(16-18-8-3-2-4-9-18)27(38)35(29)22(17-39-29)25(37)33-21(11-7-15-32-28(30)31)24(36)26-34-20-10-5-6-12-23(20)40-26/h2-6,8-10,12,19,21-22H,7,11,13-17H2,1H3,(H,33,37)(H4,30,31,32)/p+1/t19-,21?,22-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human thrombin. |
Bioorg Med Chem Lett 9: 913-8 (1999)
BindingDB Entry DOI: 10.7270/Q2V98780 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50452138

(CHEMBL2372196)Show SMILES CC(O)=O.CC(C)C[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](CCCCNC1=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C32H44N6O6.C2H4O2/c1-20(2)16-26-31(43)34-15-7-6-10-25(37-29(41)24(33)17-22-11-13-23(39)14-12-22)30(42)35-19-28(40)36-27(32(44)38-26)18-21-8-4-3-5-9-21;1-2(3)4/h3-5,8-9,11-14,20,24-27,39H,6-7,10,15-19,33H2,1-2H3,(H,34,43)(H,35,42)(H,36,40)(H,37,41)(H,38,44);1H3,(H,3,4)/t24-,25+,26+,27-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H][D-Ala2,D-Leu5]enkephalin binding to Opioid receptor delta 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072539
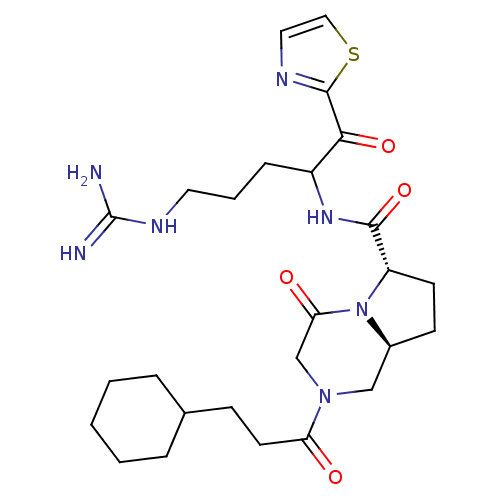
((6S,8aS)-2-(3-Cyclohexyl-propionyl)-4-oxo-octahydr...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCC1CCCCC1)C(=O)c1nccs1 Show InChI InChI=1S/C26H39N7O4S/c27-26(28)30-12-4-7-19(23(36)25-29-13-14-38-25)31-24(37)20-10-9-18-15-32(16-22(35)33(18)20)21(34)11-8-17-5-2-1-3-6-17/h13-14,17-20H,1-12,15-16H2,(H,31,37)(H4,27,28,30)/t18-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072540

((6S,8aS)-2-Benzoyl-4-oxo-octahydro-pyrrolo[1,2-a]p...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)c1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C24H29N7O4S/c25-24(26)28-10-4-7-17(20(33)22-27-11-12-36-22)29-21(34)18-9-8-16-13-30(14-19(32)31(16)18)23(35)15-5-2-1-3-6-15/h1-3,5-6,11-12,16-18H,4,7-10,13-14H2,(H,29,34)(H4,25,26,28)/t16-,17?,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H][D-Ala2,D-Leu5]enkephalin binding to Opioid receptor delta 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]naloxone to Opioid receptor mu 1 in the rat brain homogenate |
J Med Chem 25: 1432-8 (1983)
BindingDB Entry DOI: 10.7270/Q2HQ40G9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































