

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50369460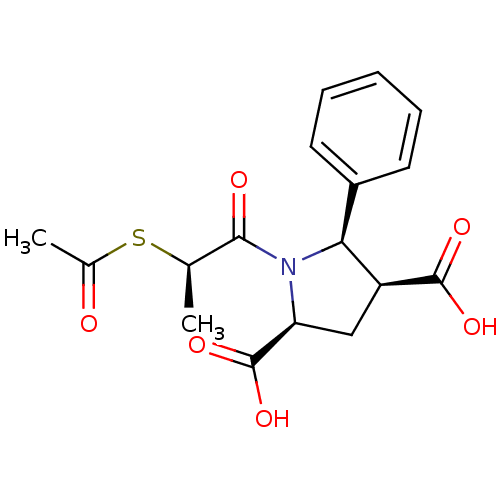 (CHEMBL1788109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Enzyme inhibitory activity towards Angiotensin I converting enzyme | J Med Chem 42: 3743-78 (1999) BindingDB Entry DOI: 10.7270/Q22Z167W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM21842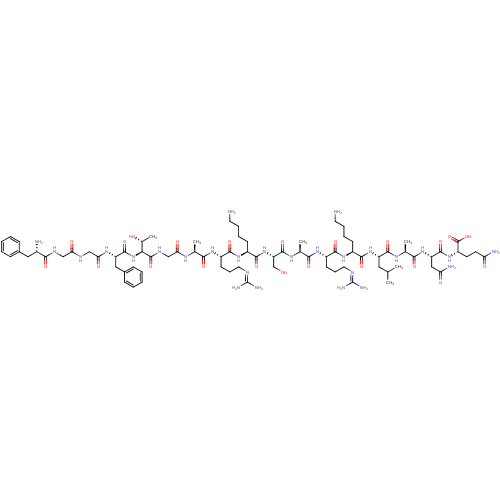 ((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM85194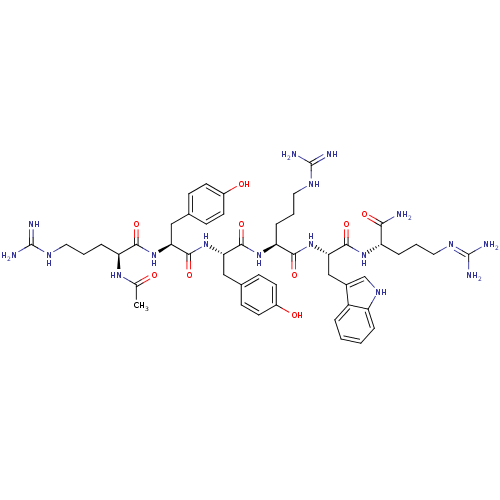 (Ac-RYYRWR-NH2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM85191 (Ac-RYYRWK-NH2 | CAS_200959-47-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM85193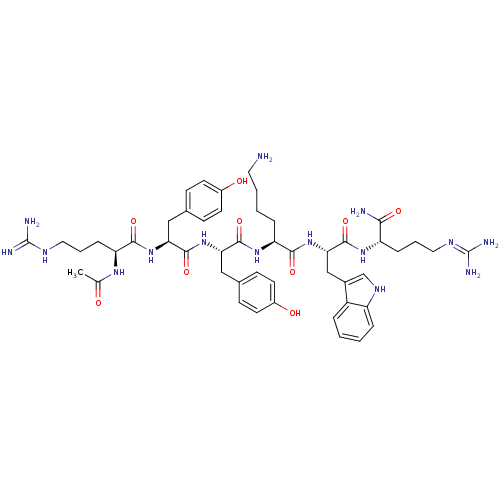 (Ac-RYYKWR-NH2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM85192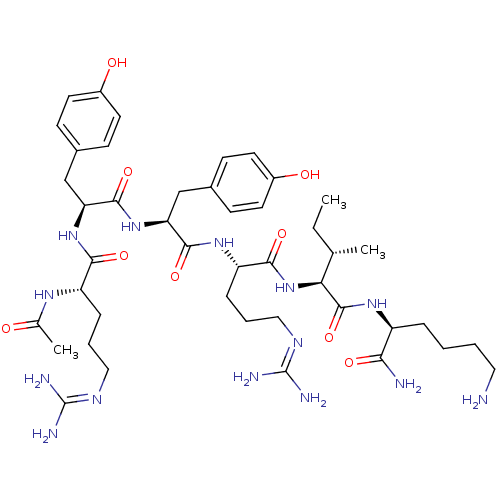 (Ac-RYYRIK-NH2 | CAS_200959-48-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50094772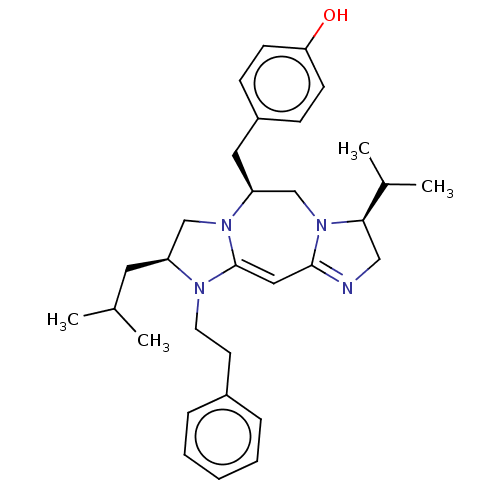 (CHEMBL3589796) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cortices and cerebella by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094772 (CHEMBL3589796) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094764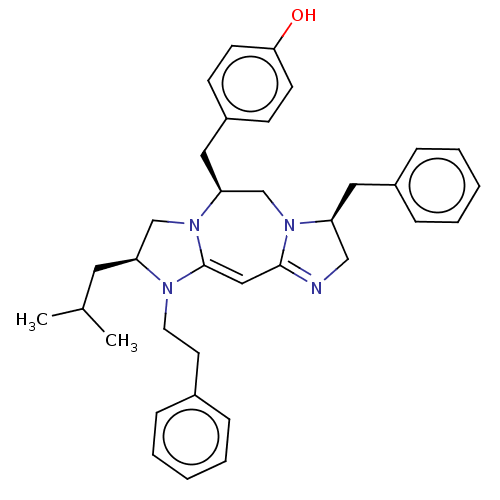 (CHEMBL3589705) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094774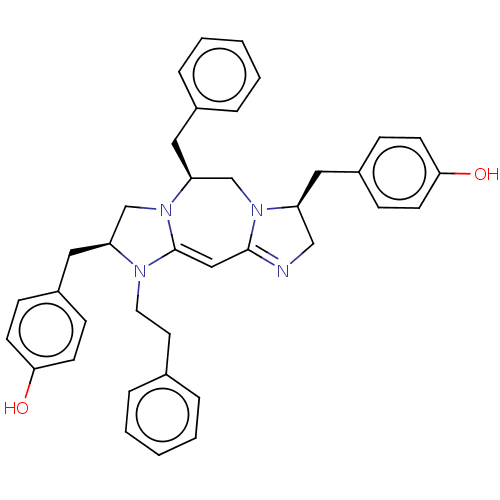 (CHEMBL3589798) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094763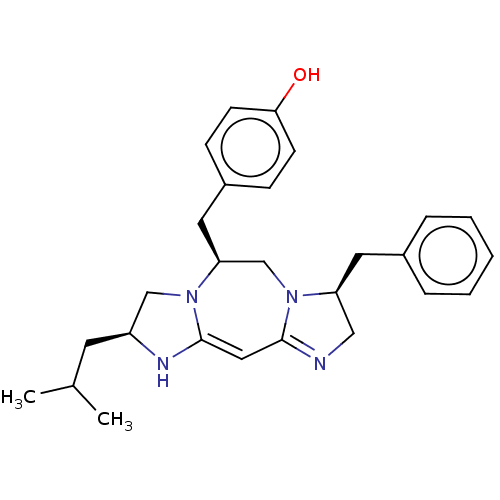 (CHEMBL3589704) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50101997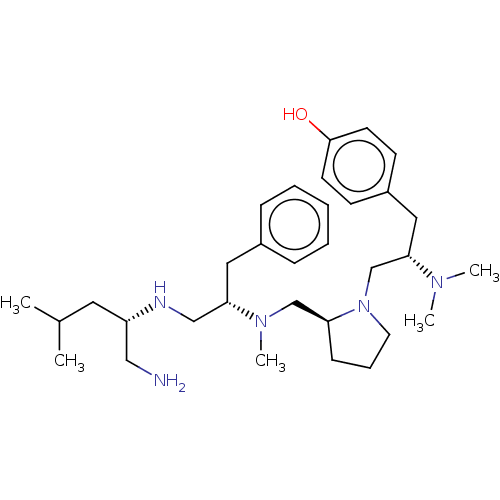 (CHEMBL3326769) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane by liquid scintillation counting analysis | Bioorg Med Chem Lett 24: 4482-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.090 BindingDB Entry DOI: 10.7270/Q26T0PDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094773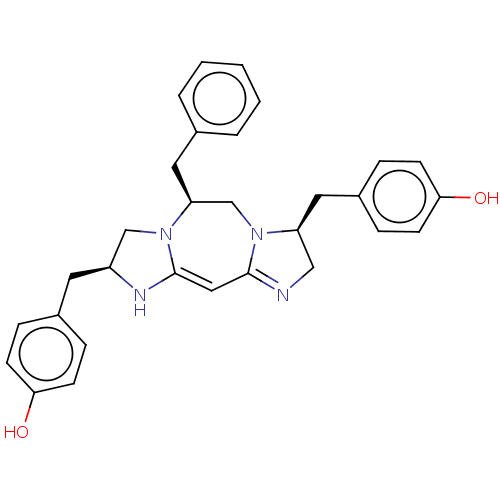 (CHEMBL3589797) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094766 (CHEMBL3589707) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50094771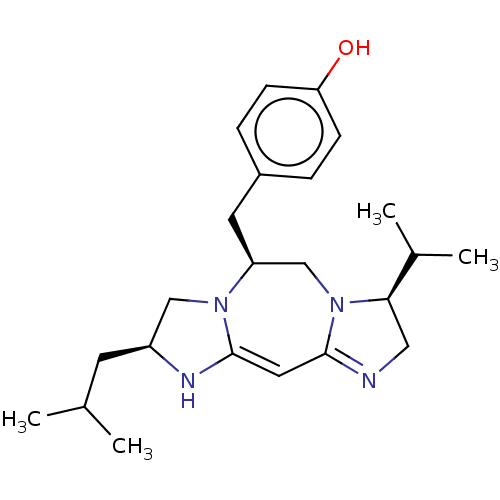 (CHEMBL3589795) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cortices and cerebella by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094771 (CHEMBL3589795) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094776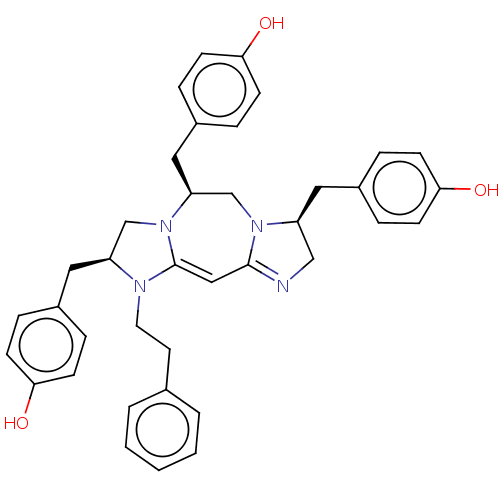 (CHEMBL3589800) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50101998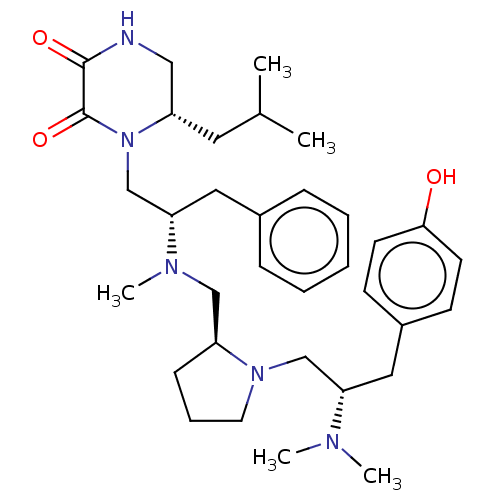 (CHEMBL3326768) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane by liquid scintillation counting analysis | Bioorg Med Chem Lett 24: 4482-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.090 BindingDB Entry DOI: 10.7270/Q26T0PDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094775 (CHEMBL3589799) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094765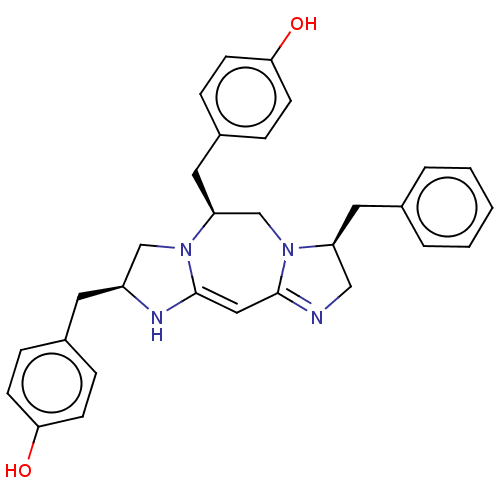 (CHEMBL3589706) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 338 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094776 (CHEMBL3589800) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 392 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094774 (CHEMBL3589798) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50102027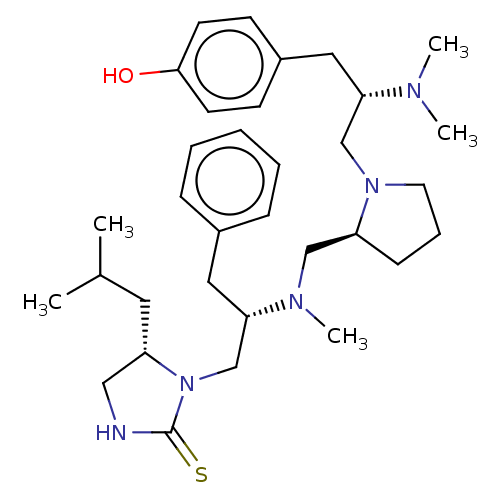 (CHEMBL3326767) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane by liquid scintillation counting analysis | Bioorg Med Chem Lett 24: 4482-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.090 BindingDB Entry DOI: 10.7270/Q26T0PDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094775 (CHEMBL3589799) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 552 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094761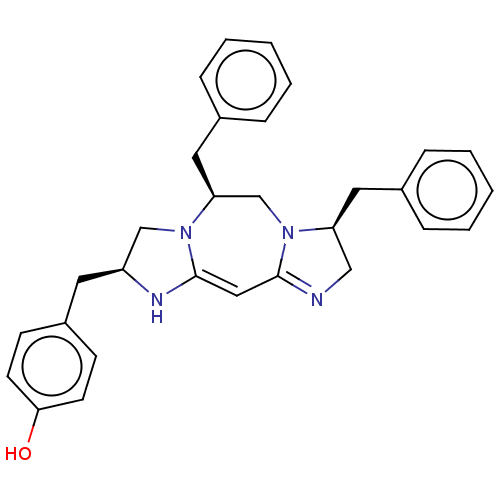 (CHEMBL3589702) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 566 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094773 (CHEMBL3589797) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 605 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50094764 (CHEMBL3589705) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 642 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cortices and cerebella by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094772 (CHEMBL3589796) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 721 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50102073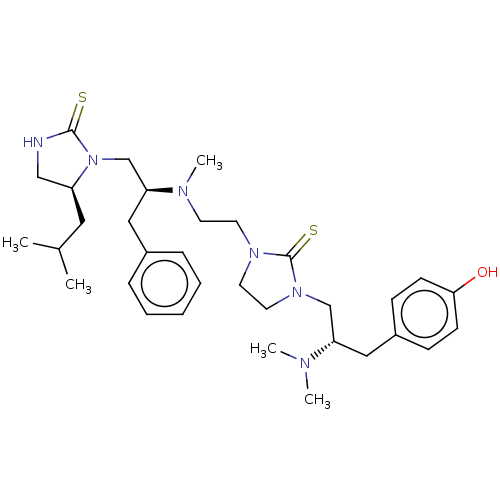 (CHEMBL3326662) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane by liquid scintillation counting analysis | Bioorg Med Chem Lett 24: 4482-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.090 BindingDB Entry DOI: 10.7270/Q26T0PDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50094774 (CHEMBL3589798) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 767 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cortices and cerebella by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094766 (CHEMBL3589707) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 878 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094764 (CHEMBL3589705) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 949 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094768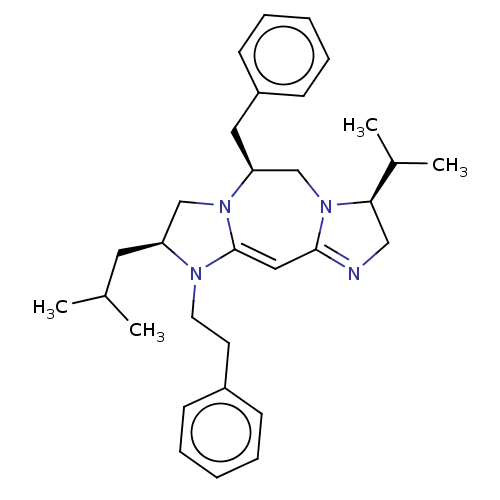 (CHEMBL3589709) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50094767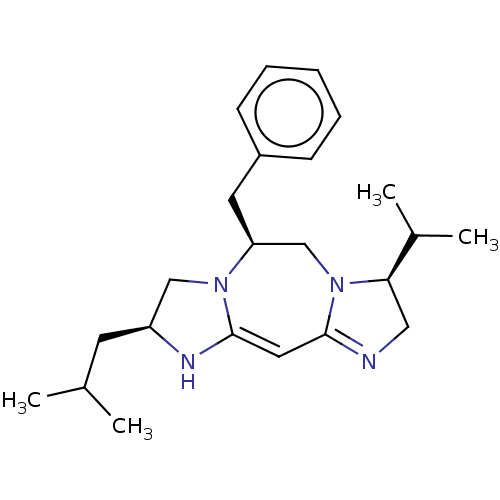 (CHEMBL3589708) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cortices and cerebella by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50102030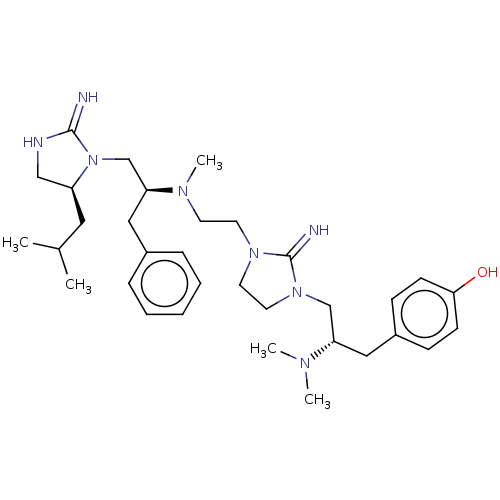 (CHEMBL3326663) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane by liquid scintillation counting analysis | Bioorg Med Chem Lett 24: 4482-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.090 BindingDB Entry DOI: 10.7270/Q26T0PDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094770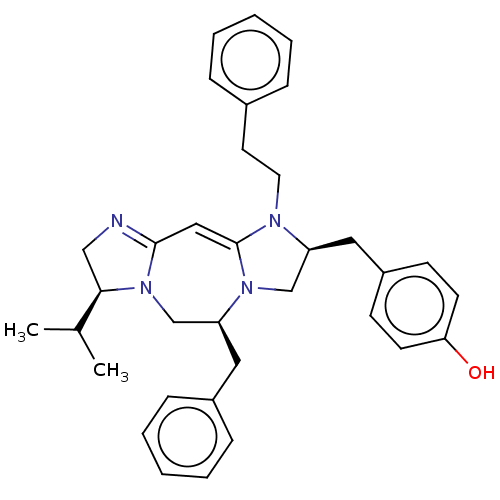 (CHEMBL3589794) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102095 (CHEMBL3326659) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cortices membrane by liquid scintillation counting analysis | Bioorg Med Chem Lett 24: 4482-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.090 BindingDB Entry DOI: 10.7270/Q26T0PDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50102029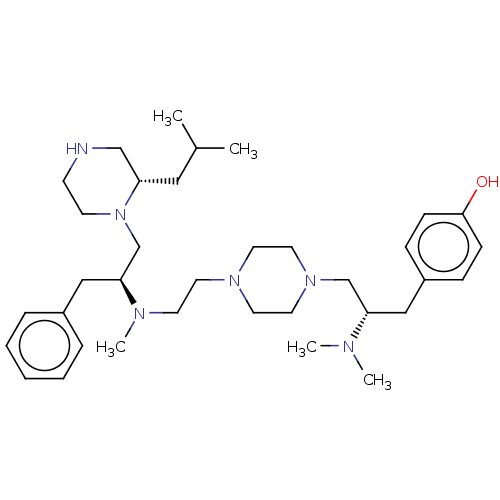 (CHEMBL3326664) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane by liquid scintillation counting analysis | Bioorg Med Chem Lett 24: 4482-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.090 BindingDB Entry DOI: 10.7270/Q26T0PDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094765 (CHEMBL3589706) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094771 (CHEMBL3589795) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50101997 (CHEMBL3326769) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cortices membrane by liquid scintillation counting analysis | Bioorg Med Chem Lett 24: 4482-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.090 BindingDB Entry DOI: 10.7270/Q26T0PDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094762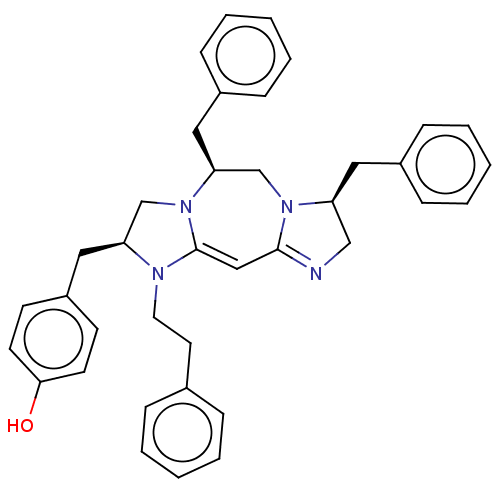 (CHEMBL3589703) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50094766 (CHEMBL3589707) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cortices and cerebella by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094763 (CHEMBL3589704) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094770 (CHEMBL3589794) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50094768 (CHEMBL3589709) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cortices and cerebella by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50102095 (CHEMBL3326659) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane by liquid scintillation counting analysis | Bioorg Med Chem Lett 24: 4482-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.090 BindingDB Entry DOI: 10.7270/Q26T0PDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50094776 (CHEMBL3589800) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cortices and cerebella by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50101994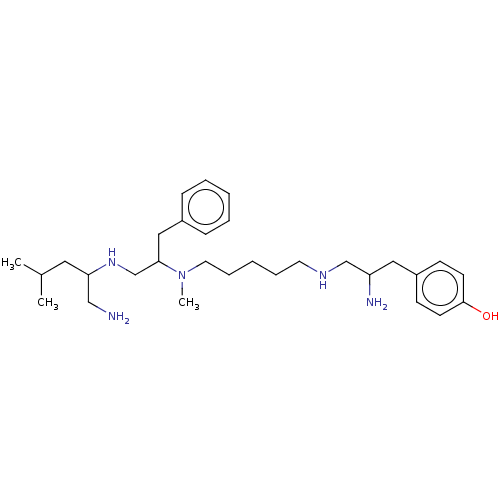 (CHEMBL3326772) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cortices membrane by liquid scintillation counting analysis | Bioorg Med Chem Lett 24: 4482-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.090 BindingDB Entry DOI: 10.7270/Q26T0PDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50101994 (CHEMBL3326772) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Monastir Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane by liquid scintillation counting analysis | Bioorg Med Chem Lett 24: 4482-5 (2014) Article DOI: 10.1016/j.bmcl.2014.07.090 BindingDB Entry DOI: 10.7270/Q26T0PDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 485 total ) | Next | Last >> |