 Found 149 hits with Last Name = 'du jardin' and Initial = 'm'
Found 149 hits with Last Name = 'du jardin' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC11 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC10 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC4 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC5 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50304783
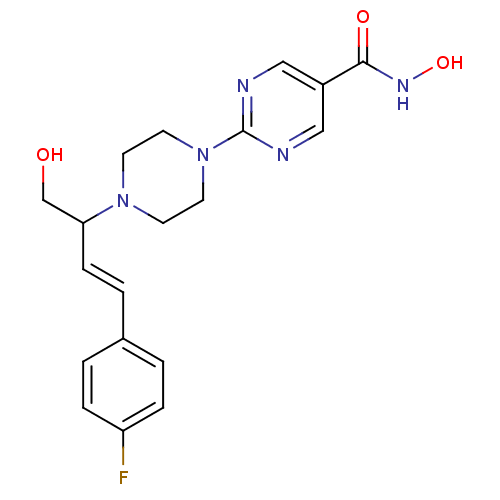
((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC2 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC3 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC2 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC11 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC5 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC9 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC3 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC9 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC10 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC7 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC7 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC4 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50304783

((+)-2-(4-(4-(4-fluorophenyl)-1-hydroxybut-3-en-2-y...)Show SMILES OCC(\C=C\c1ccc(F)cc1)N1CCN(CC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C19H22FN5O3/c20-16-4-1-14(2-5-16)3-6-17(13-26)24-7-9-25(10-8-24)19-21-11-15(12-22-19)18(27)23-28/h1-6,11-12,17,26,28H,7-10,13H2,(H,23,27)/b6-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648376
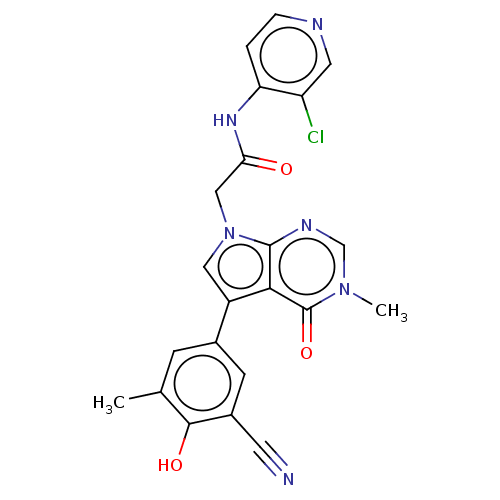
(acsmedchemlett.2c00502 OICR 10525D, 29)Show SMILES Cc1cc(cc(C#N)c1O)-c1cn(CC(=O)Nc2ccncc2Cl)c2ncn(C)c(=O)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648377
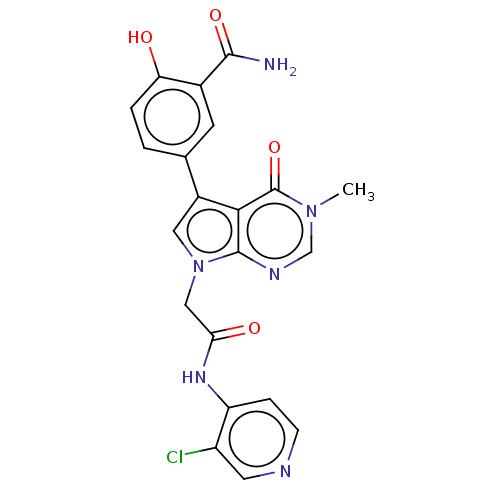
(acsmedchemlett.2c00502 OICR 9483A, 30)Show SMILES Cn1cnc2n(CC(=O)Nc3ccncc3Cl)cc(-c3ccc(O)c(c3)C(N)=O)c2c1=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648372

(acsmedchemlett.2c00502 OICR 9451A, 25)Show SMILES Cn1cnc2n(CC(=O)Nc3ccncc3Cl)cc(-c3ccc(O)c(Cl)c3)c2c1=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648371
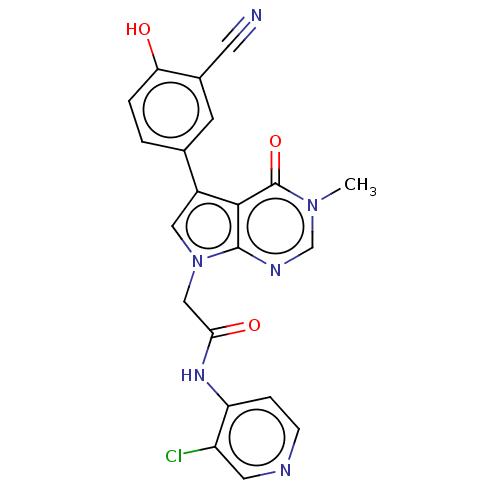
(acsmedchemlett.2c00502 OICR 9320D, 24)Show SMILES Cn1cnc2n(CC(=O)Nc3ccncc3Cl)cc(-c3ccc(O)c(c3)C#N)c2c1=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648373
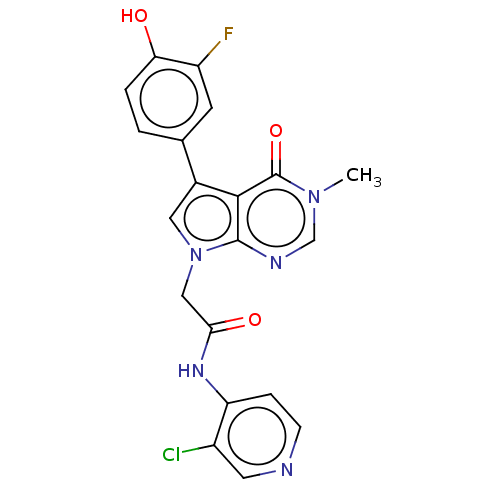
(acsmedchemlett.2c00502 OICR 9453A, 26)Show SMILES Cn1cnc2n(CC(=O)Nc3ccncc3Cl)cc(-c3ccc(O)c(F)c3)c2c1=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648374
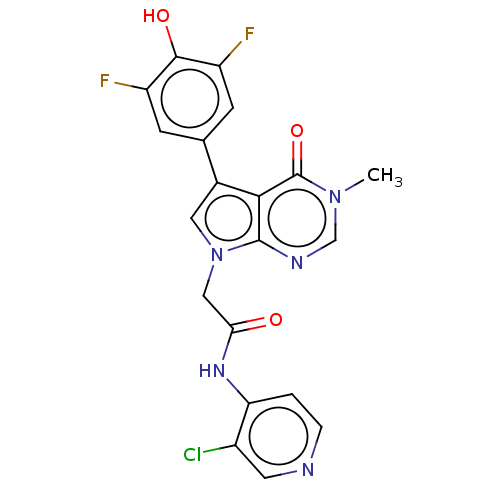
(acsmedchemlett.2c00502 OICR 9452A, 27)Show SMILES Cn1cnc2n(CC(=O)Nc3ccncc3Cl)cc(-c3cc(F)c(O)c(F)c3)c2c1=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648375
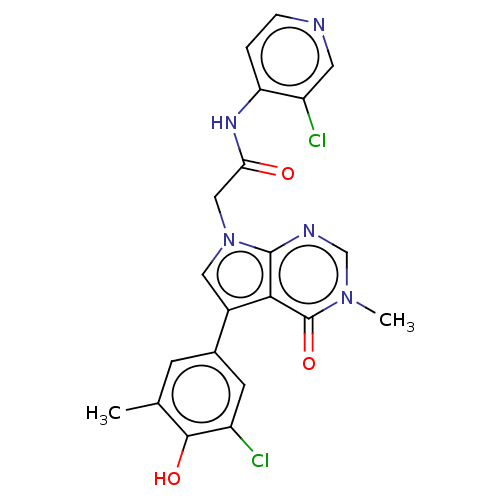
(acsmedchemlett.2c00502 Compound 28)Show SMILES Cc1cc(cc(Cl)c1O)-c1cn(CC(=O)Nc2ccncc2Cl)c2ncn(C)c(=O)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648369
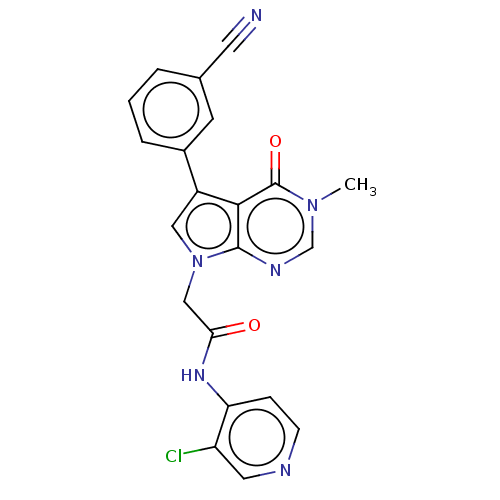
(acsmedchemlett.2c00502 OICR 9058A, 22)Show SMILES Cn1cnc2n(CC(=O)Nc3ccncc3Cl)cc(-c3cccc(c3)C#N)c2c1=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648368
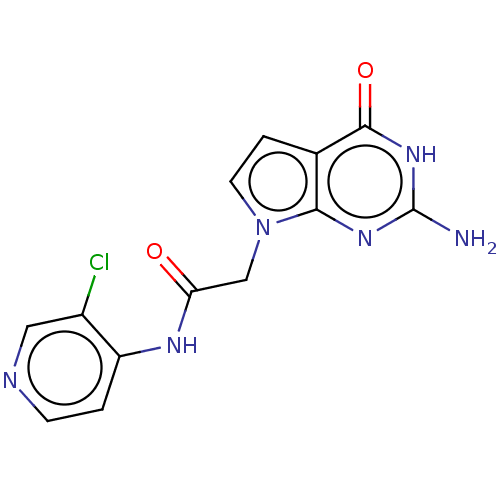
(acsmedchemlett.2c00502 OICR 7859A, 21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648364
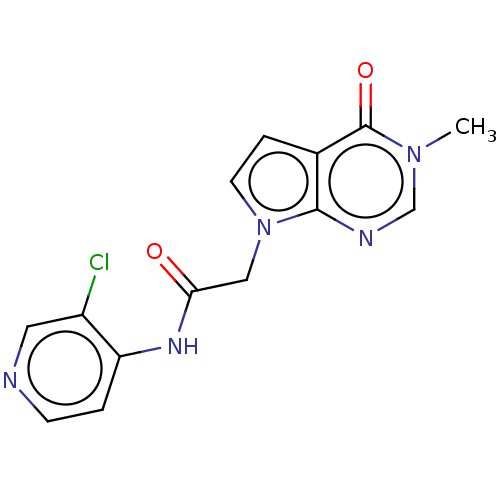
(acsmedchemlett.2c00502 OICR 7629A, 17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648370
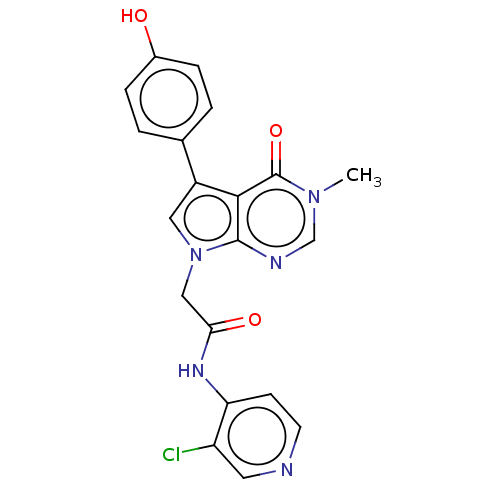
(acsmedchemlett.2c00502 OICR 9398A, 23)Show SMILES Cn1cnc2n(CC(=O)Nc3ccncc3Cl)cc(-c3ccc(O)cc3)c2c1=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648365
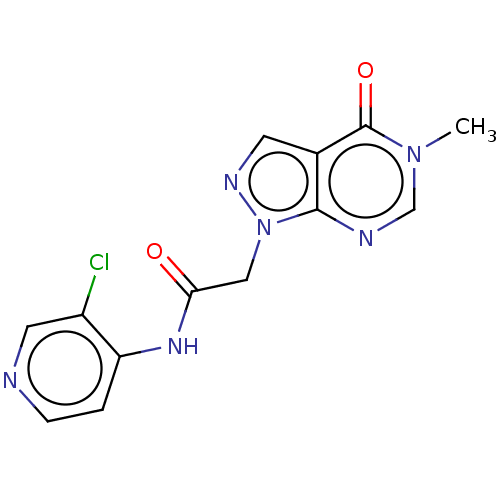
(acsmedchemlett.2c00502 OICR 7780A, 18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648358
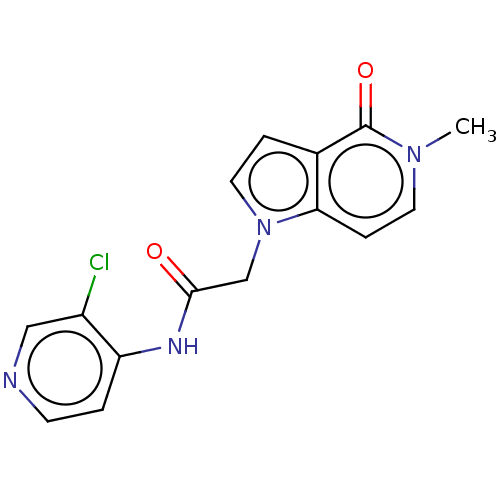
(acsmedchemlett.2c00502 OICR 4425A, 11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648359
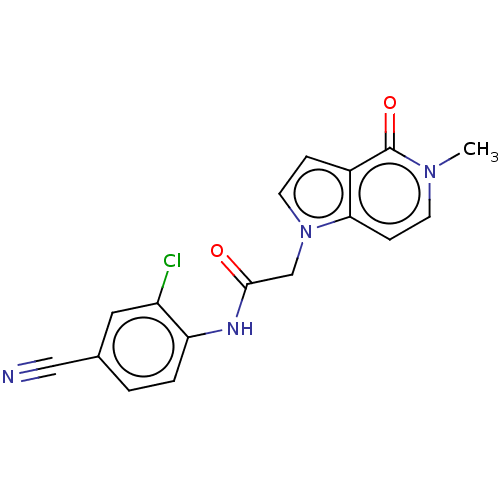
(acsmedchemlett.2c00502 OICR 7510A, 12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648355
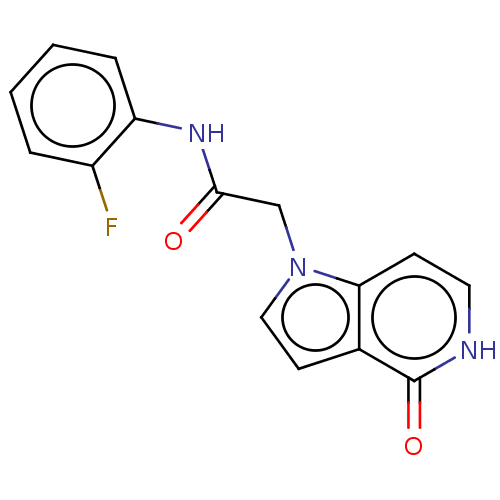
(acsmedchemlett.2c00502 OICR 4349A, 8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648354
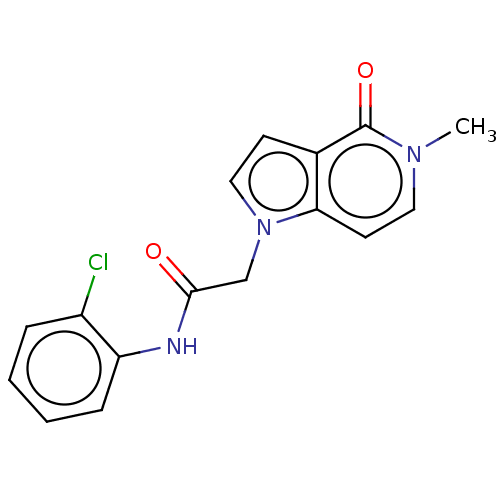
(acsmedchemlett.2c00502 OICR 4186A, 7) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648360
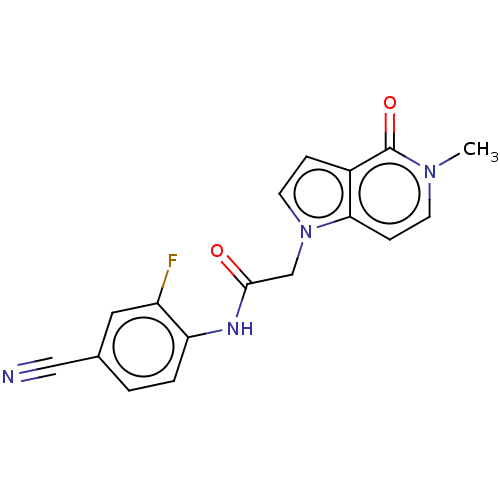
(acsmedchemlett.2c00502 OICR 7511A, 13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648356
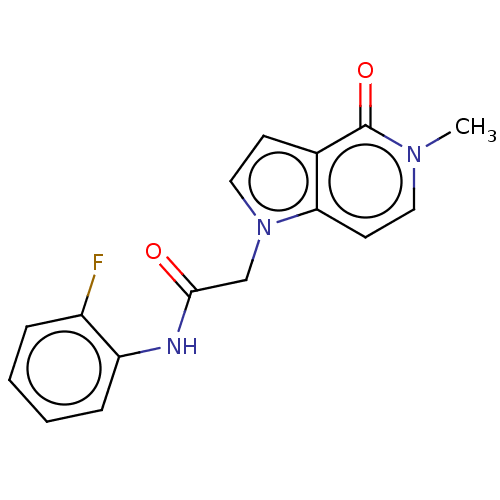
(acsmedchemlett.2c00502 OICR 7508A, 9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648353
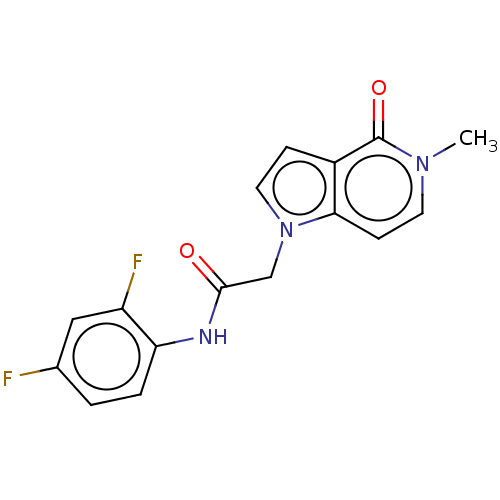
(acsmedchemlett.2c00502 OICR 3428A, 6) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648363
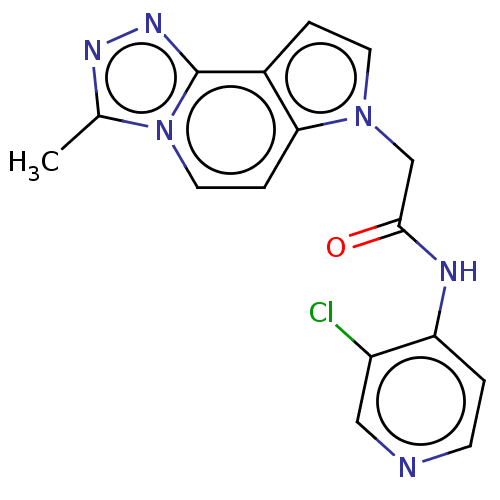
(acsmedchemlett.2c00502 OICR 7711A, 16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648366
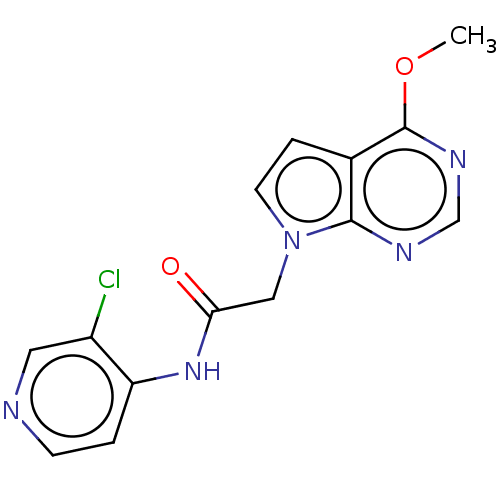
(acsmedchemlett.2c00502 OICR 7821A, 19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648362
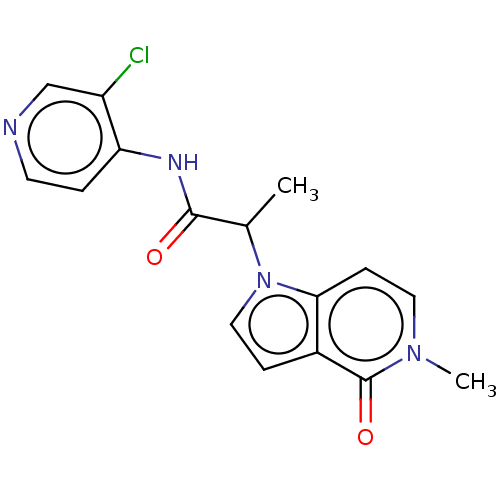
(acsmedchemlett.2c00502 OICR 7685A, 15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648361
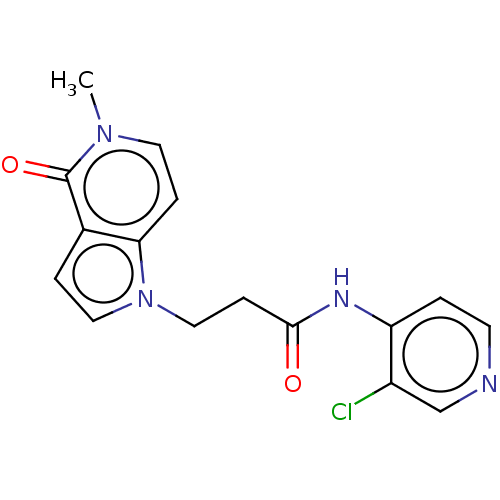
(acsmedchemlett.2c00502 OICR 7662A, 14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648367
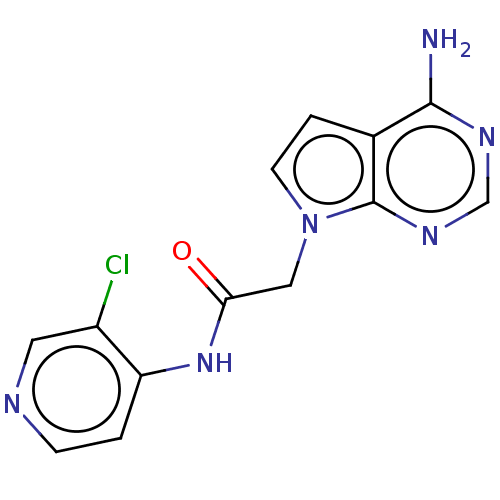
(acsmedchemlett.2c00502 OICR 8312A, 20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648357
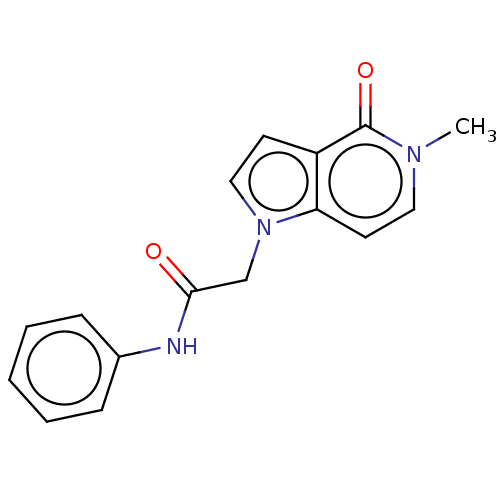
(acsmedchemlett.2c00502 Compound 10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648290
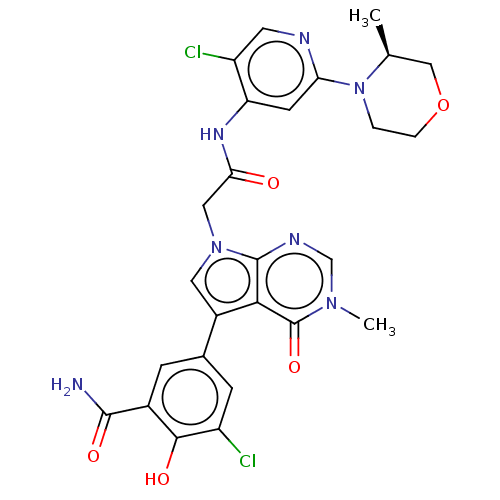
(OICR 11029A)Show SMILES C[C@H]1COCCN1c1cc(NC(=O)Cn2cc(-c3cc(Cl)c(O)c(c3)C(N)=O)c3c2ncn(C)c3=O)c(Cl)cn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of Btk, biotinylated SH2 peptide substrate (Src... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648292
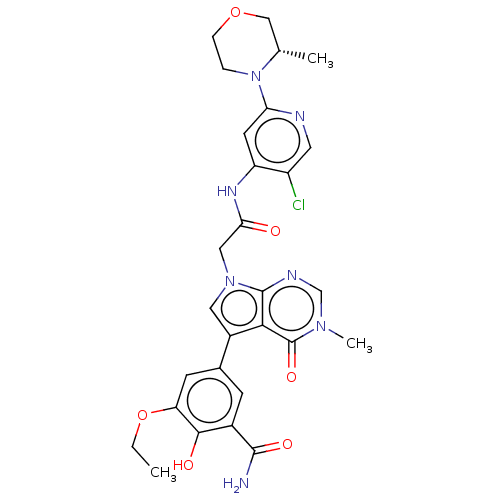
(OICR 11600D)Show SMILES CCOc1cc(cc(C(N)=O)c1O)-c1cn(CC(=O)Nc2cc(ncc2Cl)N2CCOC[C@@H]2C)c2ncn(C)c(=O)c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 4.11E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of Btk, biotinylated SH2 peptide substrate (Src... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein [5-128]
(Homo sapiens (Human)) | BDBM648353

(acsmedchemlett.2c00502 OICR 3428A, 6) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | 2.82E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data