 Found 53 hits with Last Name = 'dumont' and Initial = 'fj'
Found 53 hits with Last Name = 'dumont' and Initial = 'fj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079775
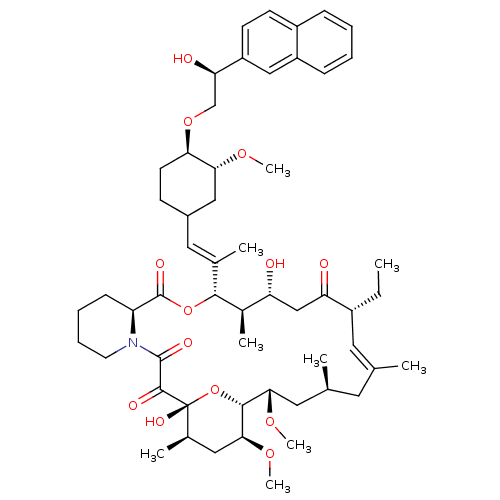
(17-Ethyl-1,14-dihydroxy-12-{2-[4-(2-hydroxy-2-naph...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2ccc3ccccc3c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C55H79NO13/c1-10-38-24-32(2)23-33(3)25-48(65-8)51-49(66-9)27-35(5)55(63,69-51)52(60)53(61)56-22-14-13-17-42(56)54(62)68-50(36(6)43(57)30-44(38)58)34(4)26-37-18-21-46(47(28-37)64-7)67-31-45(59)41-20-19-39-15-11-12-16-40(39)29-41/h11-12,15-16,19-20,24,26,29,33,35-38,42-43,45-51,57,59,63H,10,13-14,17-18,21-23,25,27-28,30-31H2,1-9H3/b32-24+,34-26+/t33-,35+,36+,37?,38+,42-,43+,45+,46+,47+,48-,49-,50+,51+,55+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079784
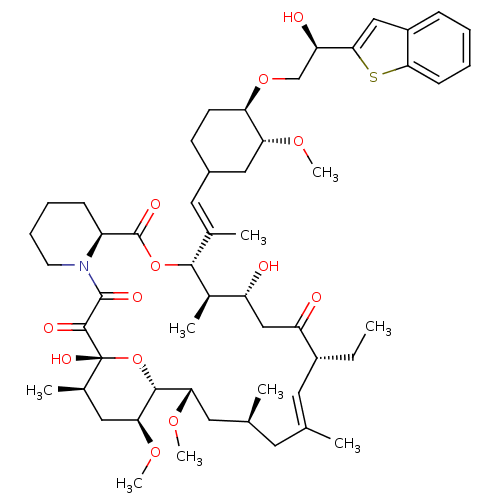
(12-{2-[4-(2-Benzo[b]thiophen-2-yl-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2cc3ccccc3s2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C53H77NO13S/c1-10-36-22-30(2)21-31(3)23-44(63-8)49-45(64-9)25-33(5)53(61,67-49)50(58)51(59)54-20-14-13-16-38(54)52(60)66-48(34(6)39(55)28-40(36)56)32(4)24-35-18-19-42(43(26-35)62-7)65-29-41(57)47-27-37-15-11-12-17-46(37)68-47/h11-12,15,17,22,24,27,31,33-36,38-39,41-45,48-49,55,57,61H,10,13-14,16,18-21,23,25-26,28-29H2,1-9H3/b30-22+,32-24+/t31-,33+,34+,35?,36+,38-,39+,41+,42+,43+,44-,45-,48+,49+,53+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079783
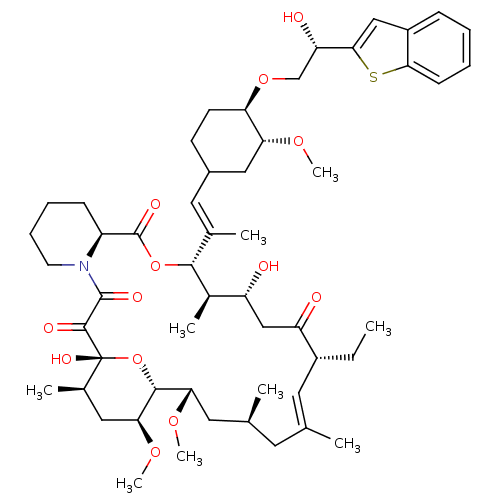
(12-{2-[4-(2-Benzo[b]thiophen-2-yl-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2cc3ccccc3s2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C53H77NO13S/c1-10-36-22-30(2)21-31(3)23-44(63-8)49-45(64-9)25-33(5)53(61,67-49)50(58)51(59)54-20-14-13-16-38(54)52(60)66-48(34(6)39(55)28-40(36)56)32(4)24-35-18-19-42(43(26-35)62-7)65-29-41(57)47-27-37-15-11-12-17-46(37)68-47/h11-12,15,17,22,24,27,31,33-36,38-39,41-45,48-49,55,57,61H,10,13-14,16,18-21,23,25-26,28-29H2,1-9H3/b30-22+,32-24+/t31-,33+,34+,35?,36+,38-,39+,41-,42+,43+,44-,45-,48+,49+,53+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079776

(17-Ethyl-1,14-dihydroxy-12-(2-{4-[2-hydroxy-2-(3-t...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2cccc(c2)C(F)(F)F)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C52H76F3NO13/c1-10-35-21-29(2)20-30(3)22-44(65-8)47-45(66-9)24-32(5)51(63,69-47)48(60)49(61)56-19-12-11-16-38(56)50(62)68-46(33(6)39(57)27-40(35)58)31(4)23-34-17-18-42(43(25-34)64-7)67-28-41(59)36-14-13-15-37(26-36)52(53,54)55/h13-15,21,23,26,30,32-35,38-39,41-47,57,59,63H,10-12,16-20,22,24-25,27-28H2,1-9H3/b29-21+,31-23+/t30-,32+,33+,34?,35+,38-,39+,41+,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079782
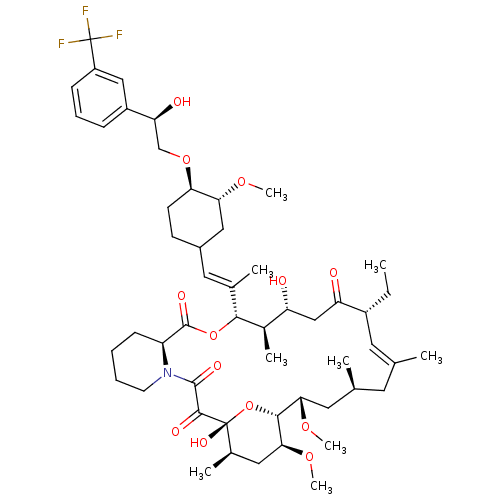
(17-Ethyl-1,14-dihydroxy-12-(2-{4-[2-hydroxy-2-(3-t...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2cccc(c2)C(F)(F)F)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C52H76F3NO13/c1-10-35-21-29(2)20-30(3)22-44(65-8)47-45(66-9)24-32(5)51(63,69-47)48(60)49(61)56-19-12-11-16-38(56)50(62)68-46(33(6)39(57)27-40(35)58)31(4)23-34-17-18-42(43(25-34)64-7)67-28-41(59)36-14-13-15-37(26-36)52(53,54)55/h13-15,21,23,26,30,32-35,38-39,41-47,57,59,63H,10-12,16-20,22,24-25,27-28H2,1-9H3/b29-21+,31-23+/t30-,32+,33+,34?,35+,38-,39+,41-,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079770

(C32-O-Phenalkyl ether derivative of Ascomycin | CH...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OCCc2ccccc2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C51H77NO12/c1-10-38-25-31(2)24-32(3)26-44(60-8)47-45(61-9)28-34(5)51(58,64-47)48(55)49(56)52-22-15-14-18-39(52)50(57)63-46(35(6)40(53)30-41(38)54)33(4)27-37-19-20-42(43(29-37)59-7)62-23-21-36-16-12-11-13-17-36/h11-13,16-17,25,27,32,34-35,37-40,42-47,53,58H,10,14-15,18-24,26,28-30H2,1-9H3/b31-25+,33-27+/t32-,34+,35+,37-,38+,39-,40-,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of serine/threonine protein phosphatase calcineurin (CAN) |
Bioorg Med Chem Lett 9: 2085-8 (1999)
BindingDB Entry DOI: 10.7270/Q22806SS |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079779
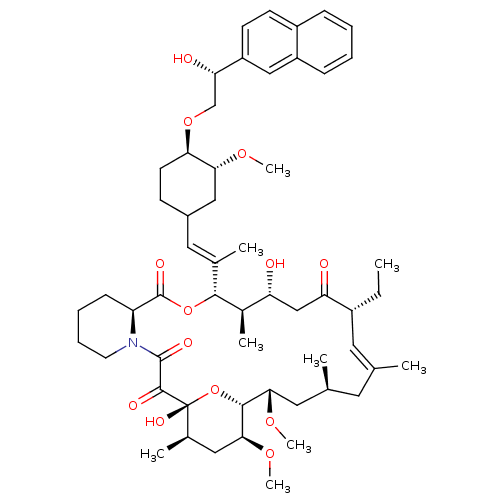
(17-Ethyl-1,14-dihydroxy-12-{2-[4-(2-hydroxy-2-naph...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2ccc3ccccc3c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C55H79NO13/c1-10-38-24-32(2)23-33(3)25-48(65-8)51-49(66-9)27-35(5)55(63,69-51)52(60)53(61)56-22-14-13-17-42(56)54(62)68-50(36(6)43(57)30-44(38)58)34(4)26-37-18-21-46(47(28-37)64-7)67-31-45(59)41-20-19-39-15-11-12-16-40(39)29-41/h11-12,15-16,19-20,24,26,29,33,35-38,42-43,45-51,57,59,63H,10,13-14,17-18,21-23,25,27-28,30-31H2,1-9H3/b32-24+,34-26+/t33-,35+,36+,37?,38+,42-,43+,45-,46+,47+,48-,49-,50+,51+,55+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079778
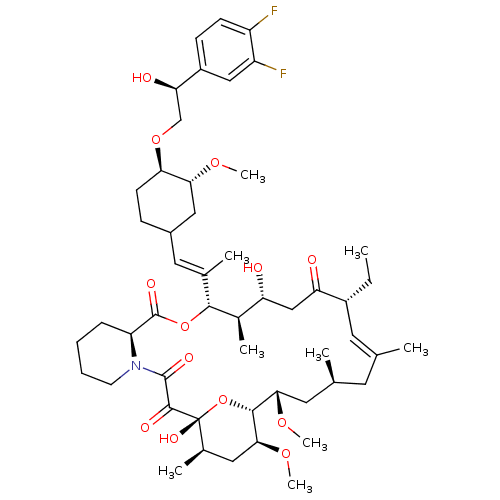
(12-(2-{4-[2-(3,4-Difluoro-phenyl)-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2ccc(F)c(F)c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C51H75F2NO13/c1-10-34-20-28(2)19-29(3)21-44(63-8)47-45(64-9)23-31(5)51(61,67-47)48(58)49(59)54-18-12-11-13-38(54)50(60)66-46(32(6)39(55)26-40(34)56)30(4)22-33-14-17-42(43(24-33)62-7)65-27-41(57)35-15-16-36(52)37(53)25-35/h15-16,20,22,25,29,31-34,38-39,41-47,55,57,61H,10-14,17-19,21,23-24,26-27H2,1-9H3/b28-20+,30-22+/t29-,31+,32+,33?,34+,38-,39+,41+,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079780
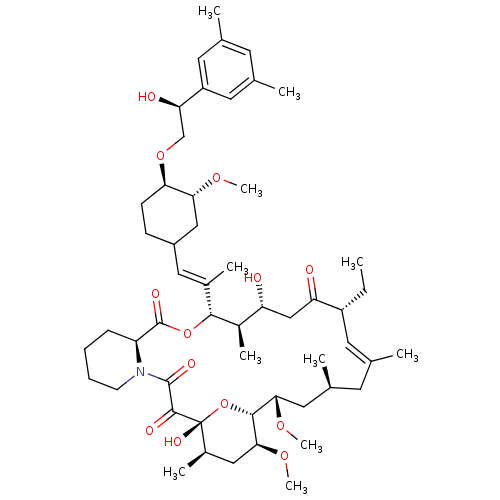
(12-(2-{4-[2-(3,5-Dimethyl-phenyl)-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2cc(C)cc(C)c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C53H81NO13/c1-12-38-21-30(2)20-33(5)24-46(63-10)49-47(64-11)26-35(7)53(61,67-49)50(58)51(59)54-18-14-13-15-40(54)52(60)66-48(36(8)41(55)28-42(38)56)34(6)25-37-16-17-44(45(27-37)62-9)65-29-43(57)39-22-31(3)19-32(4)23-39/h19,21-23,25,33,35-38,40-41,43-49,55,57,61H,12-18,20,24,26-29H2,1-11H3/b30-21+,34-25+/t33-,35+,36+,37?,38+,40-,41+,43+,44+,45+,46-,47-,48+,49+,53+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079774
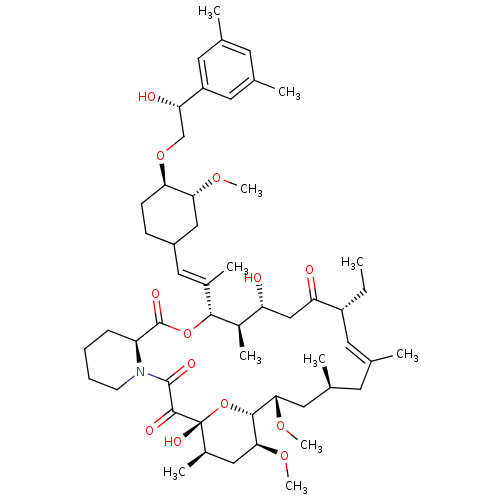
(12-(2-{4-[2-(3,5-Dimethyl-phenyl)-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2cc(C)cc(C)c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C53H81NO13/c1-12-38-21-30(2)20-33(5)24-46(63-10)49-47(64-11)26-35(7)53(61,67-49)50(58)51(59)54-18-14-13-15-40(54)52(60)66-48(36(8)41(55)28-42(38)56)34(6)25-37-16-17-44(45(27-37)62-9)65-29-43(57)39-22-31(3)19-32(4)23-39/h19,21-23,25,33,35-38,40-41,43-49,55,57,61H,12-18,20,24,26-29H2,1-11H3/b30-21+,34-25+/t33-,35+,36+,37?,38+,40-,41+,43-,44+,45+,46-,47-,48+,49+,53+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079781

(12-(2-{4-[2-(3,4-Difluoro-phenyl)-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2ccc(F)c(F)c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C51H75F2NO13/c1-10-34-20-28(2)19-29(3)21-44(63-8)47-45(64-9)23-31(5)51(61,67-47)48(58)49(59)54-18-12-11-13-38(54)50(60)66-46(32(6)39(55)26-40(34)56)30(4)22-33-14-17-42(43(24-33)62-7)65-27-41(57)35-15-16-36(52)37(53)25-35/h15-16,20,22,25,29,31-34,38-39,41-47,55,57,61H,10-14,17-19,21,23-24,26-27H2,1-9H3/b28-20+,30-22+/t29-,31+,32+,33?,34+,38-,39+,41-,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079767

(C32-O-Phenalkyl ether derivative of Ascomycin | C3...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OCCCc2ccccc2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C52H79NO12/c1-10-39-26-32(2)25-33(3)27-45(61-8)48-46(62-9)29-35(5)52(59,65-48)49(56)50(57)53-23-15-14-20-40(53)51(58)64-47(36(6)41(54)31-42(39)55)34(4)28-38-21-22-43(44(30-38)60-7)63-24-16-19-37-17-12-11-13-18-37/h11-13,17-18,26,28,33,35-36,38-41,43-48,54,59H,10,14-16,19-25,27,29-31H2,1-9H3/b32-26+,34-28+/t33-,35+,36+,38-,39+,40-,41-,43+,44+,45-,46-,47+,48+,52+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of serine/threonine protein phosphatase calcineurin (CAN) |
Bioorg Med Chem Lett 9: 2085-8 (1999)
BindingDB Entry DOI: 10.7270/Q22806SS |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50079777
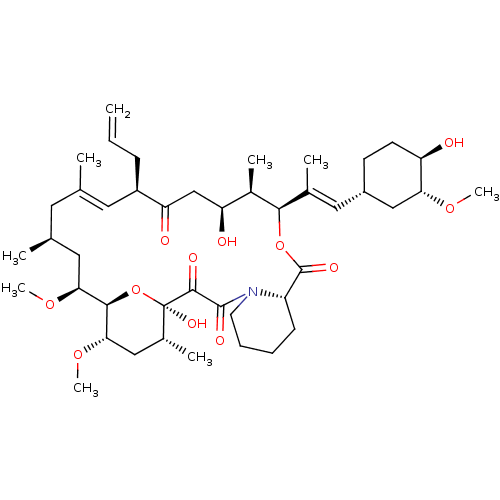
((E)-(1R,9S,12S,13R,14R,21S,23S,24R,25S,27R)-17-All...)Show SMILES [H][C@]12O[C@](O)([C@H](C)C[C@@H]1OC)C(=O)C(=O)N1CCCC[C@@]1([H])C(=O)O[C@@H]([C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)\C=C(C)\C[C@H](C)C[C@@H]2OC)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:39| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30?,31?,32-,33+,34+,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Calcineurin (CaN phosphatase) |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50071450
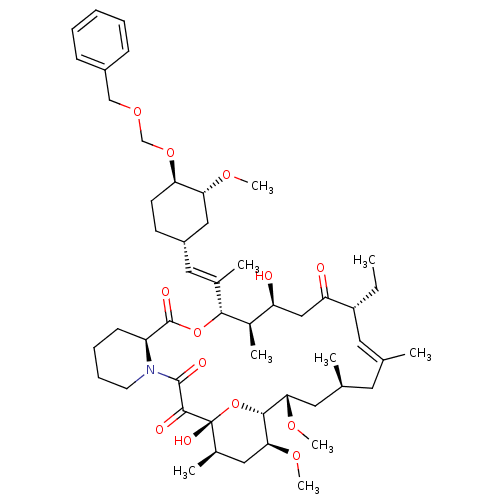
(Ascomycin derivative | CHEMBL385568)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OCOCc2ccccc2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C51H77NO13/c1-10-38-23-31(2)22-32(3)24-44(60-8)47-45(61-9)26-34(5)51(58,65-47)48(55)49(56)52-21-15-14-18-39(52)50(57)64-46(35(6)40(53)28-41(38)54)33(4)25-37-19-20-42(43(27-37)59-7)63-30-62-29-36-16-12-11-13-17-36/h11-13,16-17,23,25,32,34-35,37-40,42-47,53,58H,10,14-15,18-22,24,26-30H2,1-9H3/b31-23+,33-25+/t32-,34+,35+,37-,38+,39-,40-,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for FK506 binding protein 12 using [3H]-dihydro FK-506 radioligand was determined |
Bioorg Med Chem Lett 8: 2253-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F8K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50071463
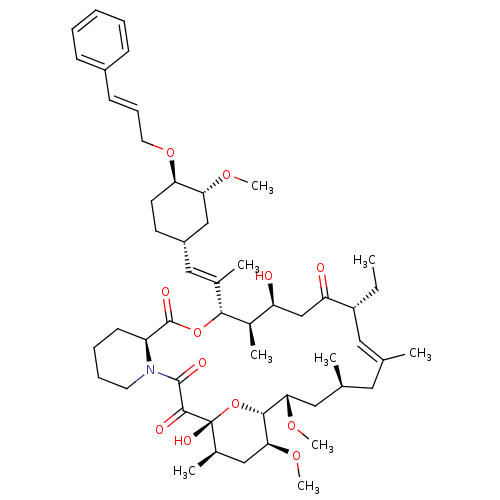
(Ascomycin derivative | C32-O-cinnamyl ether analog...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OC\C=C\c2ccccc2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C52H77NO12/c1-10-39-26-32(2)25-33(3)27-45(61-8)48-46(62-9)29-35(5)52(59,65-48)49(56)50(57)53-23-15-14-20-40(53)51(58)64-47(36(6)41(54)31-42(39)55)34(4)28-38-21-22-43(44(30-38)60-7)63-24-16-19-37-17-12-11-13-18-37/h11-13,16-19,26,28,33,35-36,38-41,43-48,54,59H,10,14-15,20-25,27,29-31H2,1-9H3/b19-16+,32-26+,34-28+/t33-,35+,36+,38-,39+,40-,41-,43+,44+,45-,46-,47+,48+,52+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for FK506 binding protein 12 using [3H]-dihydro FK-506 radioligand was determined |
Bioorg Med Chem Lett 8: 2253-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F8K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068939

((E)-(9S,12S,13R,14S,17R,21S,23S,24R,25S,27S)-17-Et...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C43H69NO12/c1-10-30-18-24(2)17-25(3)19-36(53-8)39-37(54-9)21-27(5)43(51,56-39)40(48)41(49)44-16-12-11-13-31(44)42(50)55-38(28(6)33(46)23-34(30)47)26(4)20-29-14-15-32(45)35(22-29)52-7/h18,20,25,27-33,35-39,45-46,51H,10-17,19,21-23H2,1-9H3/b24-18+,26-20+/t25-,27+,28+,29-,30+,31-,32+,33-,35+,36-,37-,38+,39+,43+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for FK506 binding protein 12 using [3H]-dihydro FK-506 radioligand was determined |
Bioorg Med Chem Lett 8: 2253-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F8K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50071466
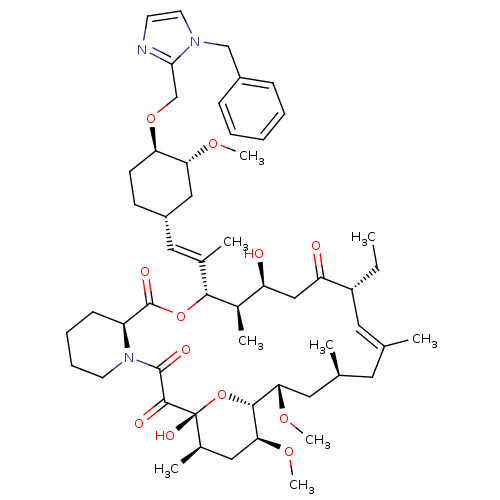
(Ascomycin derivative | CHEMBL386482)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OCc2nccn2Cc2ccccc2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C54H79N3O12/c1-10-40-25-33(2)24-34(3)26-46(65-8)50-47(66-9)28-36(5)54(63,69-50)51(60)52(61)57-22-15-14-18-41(57)53(62)68-49(37(6)42(58)30-43(40)59)35(4)27-39-19-20-44(45(29-39)64-7)67-32-48-55-21-23-56(48)31-38-16-12-11-13-17-38/h11-13,16-17,21,23,25,27,34,36-37,39-42,44-47,49-50,58,63H,10,14-15,18-20,22,24,26,28-32H2,1-9H3/b33-25+,35-27+/t34-,36+,37+,39-,40+,41-,42-,44+,45+,46-,47-,49+,50+,54+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for FK506 binding protein 12 using [3H]-dihydro FK-506 radioligand was determined |
Bioorg Med Chem Lett 8: 2253-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F8K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50071465
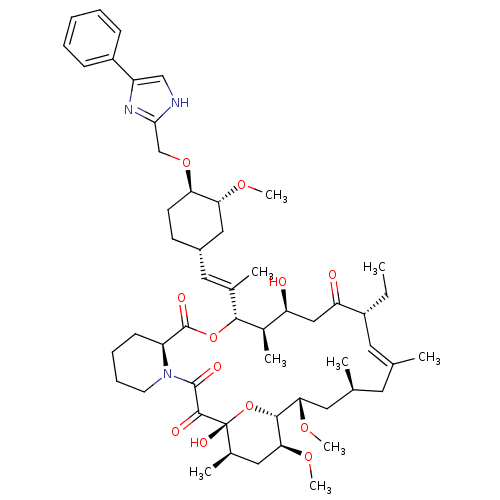
(Ascomycin derivative | CHEMBL428499)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OCc2nc(c[nH]2)-c2ccccc2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C53H77N3O12/c1-10-37-23-31(2)22-32(3)24-45(64-8)49-46(65-9)26-34(5)53(62,68-49)50(59)51(60)56-21-15-14-18-40(56)52(61)67-48(35(6)41(57)28-42(37)58)33(4)25-36-19-20-43(44(27-36)63-7)66-30-47-54-29-39(55-47)38-16-12-11-13-17-38/h11-13,16-17,23,25,29,32,34-37,40-41,43-46,48-49,57,62H,10,14-15,18-22,24,26-28,30H2,1-9H3,(H,54,55)/b31-23+,33-25+/t32-,34+,35+,36-,37+,40-,41-,43+,44+,45-,46-,48+,49+,53+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound for FK506 binding protein 12 using [3H]-dihydro FK-506 radioligand was determined |
Bioorg Med Chem Lett 8: 2253-8 (1999)
BindingDB Entry DOI: 10.7270/Q22V2F8K |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079766
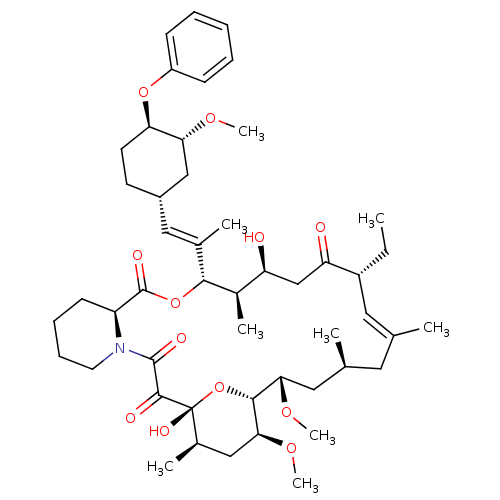
(17-Ethyl-1,14-dihydroxy-23,25-dimethoxy-12-[2-(3-m...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](Oc2ccccc2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C49H73NO12/c1-10-35-23-29(2)22-30(3)24-42(58-8)45-43(59-9)26-32(5)49(56,62-45)46(53)47(54)50-21-15-14-18-37(50)48(55)61-44(33(6)38(51)28-39(35)52)31(4)25-34-19-20-40(41(27-34)57-7)60-36-16-12-11-13-17-36/h11-13,16-17,23,25,30,32-35,37-38,40-45,51,56H,10,14-15,18-22,24,26-28H2,1-9H3/b29-23+,31-25+/t30-,32+,33+,34-,35+,37-,38-,40+,41+,42-,43-,44+,45+,49+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration determined was towards FK506 binding protein 12 by competitive binding assay using [3H]-dihydro FK-506 as radioligand |
Bioorg Med Chem Lett 9: 2085-8 (1999)
BindingDB Entry DOI: 10.7270/Q22806SS |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079767

(C32-O-Phenalkyl ether derivative of Ascomycin | C3...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OCCCc2ccccc2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C52H79NO12/c1-10-39-26-32(2)25-33(3)27-45(61-8)48-46(62-9)29-35(5)52(59,65-48)49(56)50(57)53-23-15-14-20-40(53)51(58)64-47(36(6)41(54)31-42(39)55)34(4)28-38-21-22-43(44(30-38)60-7)63-24-16-19-37-17-12-11-13-18-37/h11-13,17-18,26,28,33,35-36,38-41,43-48,54,59H,10,14-16,19-25,27,29-31H2,1-9H3/b32-26+,34-28+/t33-,35+,36+,38-,39+,40-,41-,43+,44+,45-,46-,47+,48+,52+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration determined was towards FK506 binding protein 12 by competitive binding assay using [3H]-dihydro FK-506 as radioligand |
Bioorg Med Chem Lett 9: 2085-8 (1999)
BindingDB Entry DOI: 10.7270/Q22806SS |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079765
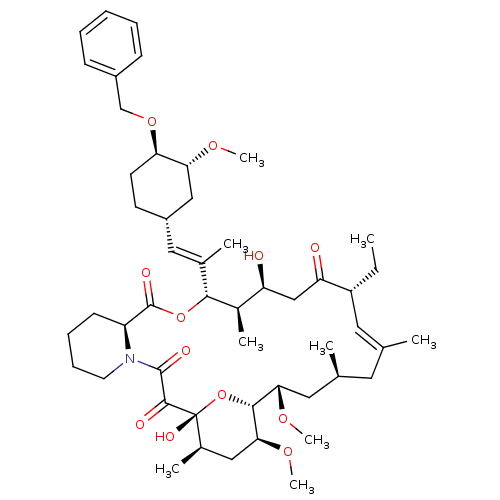
(C32-O-Phenalkyl ether derivative of Ascomycin | C3...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OCc2ccccc2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C50H75NO12/c1-10-37-23-30(2)22-31(3)24-43(59-8)46-44(60-9)26-33(5)50(57,63-46)47(54)48(55)51-21-15-14-18-38(51)49(56)62-45(34(6)39(52)28-40(37)53)32(4)25-36-19-20-41(42(27-36)58-7)61-29-35-16-12-11-13-17-35/h11-13,16-17,23,25,31,33-34,36-39,41-46,52,57H,10,14-15,18-22,24,26-29H2,1-9H3/b30-23+,32-25+/t31-,33+,34+,36-,37+,38-,39-,41+,42+,43-,44-,45+,46+,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration determined was towards FK506 binding protein 12 by competitive binding assay using [3H]-dihydro FK-506 as radioligand |
Bioorg Med Chem Lett 9: 2085-8 (1999)
BindingDB Entry DOI: 10.7270/Q22806SS |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068939

((E)-(9S,12S,13R,14S,17R,21S,23S,24R,25S,27S)-17-Et...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C43H69NO12/c1-10-30-18-24(2)17-25(3)19-36(53-8)39-37(54-9)21-27(5)43(51,56-39)40(48)41(49)44-16-12-11-13-31(44)42(50)55-38(28(6)33(46)23-34(30)47)26(4)20-29-14-15-32(45)35(22-29)52-7/h18,20,25,27-33,35-39,45-46,51H,10-17,19,21-23H2,1-9H3/b24-18+,26-20+/t25-,27+,28+,29-,30+,31-,32+,33-,35+,36-,37-,38+,39+,43+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration determined was towards FK506 binding protein 12 by competitive binding assay using [3H]-dihydro FK-506 as radioligand |
Bioorg Med Chem Lett 9: 2085-8 (1999)
BindingDB Entry DOI: 10.7270/Q22806SS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079770

(C32-O-Phenalkyl ether derivative of Ascomycin | CH...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OCCc2ccccc2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C51H77NO12/c1-10-38-25-31(2)24-32(3)26-44(60-8)47-45(61-9)28-34(5)51(58,64-47)48(55)49(56)52-22-15-14-18-39(52)50(57)63-46(35(6)40(53)30-41(38)54)33(4)27-37-19-20-42(43(29-37)59-7)62-23-21-36-16-12-11-13-17-36/h11-13,16-17,25,27,32,34-35,37-40,42-47,53,58H,10,14-15,18-24,26,28-30H2,1-9H3/b31-25+,33-27+/t32-,34+,35+,37-,38+,39-,40-,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration determined was towards FK506 binding protein 12 by competitive binding assay using [3H]-dihydro FK-506 as radioligand |
Bioorg Med Chem Lett 9: 2085-8 (1999)
BindingDB Entry DOI: 10.7270/Q22806SS |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079779

(17-Ethyl-1,14-dihydroxy-12-{2-[4-(2-hydroxy-2-naph...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2ccc3ccccc3c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C55H79NO13/c1-10-38-24-32(2)23-33(3)25-48(65-8)51-49(66-9)27-35(5)55(63,69-51)52(60)53(61)56-22-14-13-17-42(56)54(62)68-50(36(6)43(57)30-44(38)58)34(4)26-37-18-21-46(47(28-37)64-7)67-31-45(59)41-20-19-39-15-11-12-16-40(39)29-41/h11-12,15-16,19-20,24,26,29,33,35-38,42-43,45-51,57,59,63H,10,13-14,17-18,21-23,25,27-28,30-31H2,1-9H3/b32-24+,34-26+/t33-,35+,36+,37?,38+,42-,43+,45-,46+,47+,48-,49-,50+,51+,55+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against FK506 binding protein 12 using [3H]-dihydro FK-506 |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079781

(12-(2-{4-[2-(3,4-Difluoro-phenyl)-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2ccc(F)c(F)c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C51H75F2NO13/c1-10-34-20-28(2)19-29(3)21-44(63-8)47-45(64-9)23-31(5)51(61,67-47)48(58)49(59)54-18-12-11-13-38(54)50(60)66-46(32(6)39(55)26-40(34)56)30(4)22-33-14-17-42(43(24-33)62-7)65-27-41(57)35-15-16-36(52)37(53)25-35/h15-16,20,22,25,29,31-34,38-39,41-47,55,57,61H,10-14,17-19,21,23-24,26-27H2,1-9H3/b28-20+,30-22+/t29-,31+,32+,33?,34+,38-,39+,41-,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against FK506 binding protein 12 using [3H]-dihydro FK-506 |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079776

(17-Ethyl-1,14-dihydroxy-12-(2-{4-[2-hydroxy-2-(3-t...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2cccc(c2)C(F)(F)F)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C52H76F3NO13/c1-10-35-21-29(2)20-30(3)22-44(65-8)47-45(66-9)24-32(5)51(63,69-47)48(60)49(61)56-19-12-11-16-38(56)50(62)68-46(33(6)39(57)27-40(35)58)31(4)23-34-17-18-42(43(25-34)64-7)67-28-41(59)36-14-13-15-37(26-36)52(53,54)55/h13-15,21,23,26,30,32-35,38-39,41-47,57,59,63H,10-12,16-20,22,24-25,27-28H2,1-9H3/b29-21+,31-23+/t30-,32+,33+,34?,35+,38-,39+,41+,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against FK506 binding protein 12 using [3H]-dihydro FK-506 |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079774

(12-(2-{4-[2-(3,5-Dimethyl-phenyl)-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2cc(C)cc(C)c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C53H81NO13/c1-12-38-21-30(2)20-33(5)24-46(63-10)49-47(64-11)26-35(7)53(61,67-49)50(58)51(59)54-18-14-13-15-40(54)52(60)66-48(36(8)41(55)28-42(38)56)34(6)25-37-16-17-44(45(27-37)62-9)65-29-43(57)39-22-31(3)19-32(4)23-39/h19,21-23,25,33,35-38,40-41,43-49,55,57,61H,12-18,20,24,26-29H2,1-11H3/b30-21+,34-25+/t33-,35+,36+,37?,38+,40-,41+,43-,44+,45+,46-,47-,48+,49+,53+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against FK506 binding protein 12 using [3H]-dihydro FK-506 |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079780

(12-(2-{4-[2-(3,5-Dimethyl-phenyl)-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2cc(C)cc(C)c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C53H81NO13/c1-12-38-21-30(2)20-33(5)24-46(63-10)49-47(64-11)26-35(7)53(61,67-49)50(58)51(59)54-18-14-13-15-40(54)52(60)66-48(36(8)41(55)28-42(38)56)34(6)25-37-16-17-44(45(27-37)62-9)65-29-43(57)39-22-31(3)19-32(4)23-39/h19,21-23,25,33,35-38,40-41,43-49,55,57,61H,12-18,20,24,26-29H2,1-11H3/b30-21+,34-25+/t33-,35+,36+,37?,38+,40-,41+,43+,44+,45+,46-,47-,48+,49+,53+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against FK506 binding protein 12 using [3H]-dihydro FK-506 |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079782

(17-Ethyl-1,14-dihydroxy-12-(2-{4-[2-hydroxy-2-(3-t...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2cccc(c2)C(F)(F)F)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C52H76F3NO13/c1-10-35-21-29(2)20-30(3)22-44(65-8)47-45(66-9)24-32(5)51(63,69-47)48(60)49(61)56-19-12-11-16-38(56)50(62)68-46(33(6)39(57)27-40(35)58)31(4)23-34-17-18-42(43(25-34)64-7)67-28-41(59)36-14-13-15-37(26-36)52(53,54)55/h13-15,21,23,26,30,32-35,38-39,41-47,57,59,63H,10-12,16-20,22,24-25,27-28H2,1-9H3/b29-21+,31-23+/t30-,32+,33+,34?,35+,38-,39+,41-,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against FK506 binding protein 12 using [3H]-dihydro FK-506 |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079783

(12-{2-[4-(2-Benzo[b]thiophen-2-yl-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@H](O)c2cc3ccccc3s2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C53H77NO13S/c1-10-36-22-30(2)21-31(3)23-44(63-8)49-45(64-9)25-33(5)53(61,67-49)50(58)51(59)54-20-14-13-16-38(54)52(60)66-48(34(6)39(55)28-40(36)56)32(4)24-35-18-19-42(43(26-35)62-7)65-29-41(57)47-27-37-15-11-12-17-46(37)68-47/h11-12,15,17,22,24,27,31,33-36,38-39,41-45,48-49,55,57,61H,10,13-14,16,18-21,23,25-26,28-29H2,1-9H3/b30-22+,32-24+/t31-,33+,34+,35?,36+,38-,39+,41-,42+,43+,44-,45-,48+,49+,53+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against FK506 binding protein 12 using [3H]-dihydro FK-506 |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079775

(17-Ethyl-1,14-dihydroxy-12-{2-[4-(2-hydroxy-2-naph...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2ccc3ccccc3c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C55H79NO13/c1-10-38-24-32(2)23-33(3)25-48(65-8)51-49(66-9)27-35(5)55(63,69-51)52(60)53(61)56-22-14-13-17-42(56)54(62)68-50(36(6)43(57)30-44(38)58)34(4)26-37-18-21-46(47(28-37)64-7)67-31-45(59)41-20-19-39-15-11-12-16-40(39)29-41/h11-12,15-16,19-20,24,26,29,33,35-38,42-43,45-51,57,59,63H,10,13-14,17-18,21-23,25,27-28,30-31H2,1-9H3/b32-24+,34-26+/t33-,35+,36+,37?,38+,42-,43+,45+,46+,47+,48-,49-,50+,51+,55+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against FK506 binding protein 12 using [3H]-dihydro FK-506 |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079784

(12-{2-[4-(2-Benzo[b]thiophen-2-yl-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2cc3ccccc3s2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C53H77NO13S/c1-10-36-22-30(2)21-31(3)23-44(63-8)49-45(64-9)25-33(5)53(61,67-49)50(58)51(59)54-20-14-13-16-38(54)52(60)66-48(34(6)39(55)28-40(36)56)32(4)24-35-18-19-42(43(26-35)62-7)65-29-41(57)47-27-37-15-11-12-17-46(37)68-47/h11-12,15,17,22,24,27,31,33-36,38-39,41-45,48-49,55,57,61H,10,13-14,16,18-21,23,25-26,28-29H2,1-9H3/b30-22+,32-24+/t31-,33+,34+,35?,36+,38-,39+,41+,42+,43+,44-,45-,48+,49+,53+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against FK506 binding protein 12 using [3H]-dihydro FK-506 |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079777

((E)-(1R,9S,12S,13R,14R,21S,23S,24R,25S,27R)-17-All...)Show SMILES [H][C@]12O[C@](O)([C@H](C)C[C@@H]1OC)C(=O)C(=O)N1CCCC[C@@]1([H])C(=O)O[C@@H]([C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)\C=C(C)\C[C@H](C)C[C@@H]2OC)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:39| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30?,31?,32-,33+,34+,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against FK506 binding protein 12 using [3H]-dihydro FK-506 |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50079778

(12-(2-{4-[2-(3,4-Difluoro-phenyl)-2-hydroxy-ethoxy...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@H](O)CC1=O)C(\C)=C\C1CC[C@@H](OC[C@@H](O)c2ccc(F)c(F)c2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C51H75F2NO13/c1-10-34-20-28(2)19-29(3)21-44(63-8)47-45(64-9)23-31(5)51(61,67-47)48(58)49(59)54-18-12-11-13-38(54)50(60)66-46(32(6)39(55)26-40(34)56)30(4)22-33-14-17-42(43(24-33)62-7)65-27-41(57)35-15-16-36(52)37(53)25-35/h15-16,20,22,25,29,31-34,38-39,41-47,55,57,61H,10-14,17-19,21,23-24,26-27H2,1-9H3/b28-20+,30-22+/t29-,31+,32+,33?,34+,38-,39+,41+,42+,43+,44-,45-,46+,47+,51+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against FK506 binding protein 12 using [3H]-dihydro FK-506 |
Bioorg Med Chem Lett 9: 2089-94 (1999)
BindingDB Entry DOI: 10.7270/Q2XG9QBD |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284183
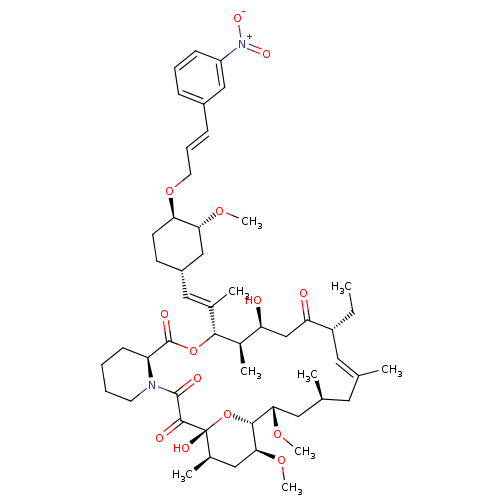
(C32-O-cinnamyl ether analogue of L-683590 | CHEMBL...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OC\C=C\c2cccc(c2)[N+]([O-])=O)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C52H76N2O14/c1-10-38-24-31(2)23-32(3)25-45(64-8)48-46(65-9)27-34(5)52(60,68-48)49(57)50(58)53-21-12-11-18-40(53)51(59)67-47(35(6)41(55)30-42(38)56)33(4)26-37-19-20-43(44(29-37)63-7)66-22-14-16-36-15-13-17-39(28-36)54(61)62/h13-17,24,26,28,32,34-35,37-38,40-41,43-48,55,60H,10-12,18-23,25,27,29-30H2,1-9H3/b16-14+,31-24+,33-26+/t32-,34+,35+,37-,38+,40-,41-,43+,44+,45-,46-,47+,48+,52+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability of the compound to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 921-926 (1994)
Article DOI: 10.1016/S0960-894X(01)80264-X
BindingDB Entry DOI: 10.7270/Q2HD7VMG |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068939

((E)-(9S,12S,13R,14S,17R,21S,23S,24R,25S,27S)-17-Et...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C43H69NO12/c1-10-30-18-24(2)17-25(3)19-36(53-8)39-37(54-9)21-27(5)43(51,56-39)40(48)41(49)44-16-12-11-13-31(44)42(50)55-38(28(6)33(46)23-34(30)47)26(4)20-29-14-15-32(45)35(22-29)52-7/h18,20,25,27-33,35-39,45-46,51H,10-17,19,21-23H2,1-9H3/b24-18+,26-20+/t25-,27+,28+,29-,30+,31-,32+,33-,35+,36-,37-,38+,39+,43+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability of the compound to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 921-926 (1994)
Article DOI: 10.1016/S0960-894X(01)80264-X
BindingDB Entry DOI: 10.7270/Q2HD7VMG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284184

(C32-O-cinnamyl ether analogue of L-683590 | CHEMBL...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OC\C=C\c2ccc(Br)cc2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C52H76BrNO12/c1-10-38-25-31(2)24-32(3)26-45(62-8)48-46(63-9)28-34(5)52(60,66-48)49(57)50(58)54-22-12-11-15-40(54)51(59)65-47(35(6)41(55)30-42(38)56)33(4)27-37-18-21-43(44(29-37)61-7)64-23-13-14-36-16-19-39(53)20-17-36/h13-14,16-17,19-20,25,27,32,34-35,37-38,40-41,43-48,55,60H,10-12,15,18,21-24,26,28-30H2,1-9H3/b14-13+,31-25+,33-27+/t32-,34+,35+,37-,38+,40-,41-,43+,44+,45-,46-,47+,48+,52+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability of the compound to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 921-926 (1994)
Article DOI: 10.1016/S0960-894X(01)80264-X
BindingDB Entry DOI: 10.7270/Q2HD7VMG |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50071451

(Ascomycin derivative | C32-O-cinnamyl ether analog...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OC\C=C\c2ccc(O)cc2)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C52H77NO13/c1-10-38-25-31(2)24-32(3)26-45(62-8)48-46(63-9)28-34(5)52(60,66-48)49(57)50(58)53-22-12-11-15-40(53)51(59)65-47(35(6)41(55)30-42(38)56)33(4)27-37-18-21-43(44(29-37)61-7)64-23-13-14-36-16-19-39(54)20-17-36/h13-14,16-17,19-20,25,27,32,34-35,37-38,40-41,43-48,54-55,60H,10-12,15,18,21-24,26,28-30H2,1-9H3/b14-13+,31-25+,33-27+/t32-,34+,35+,37-,38+,40-,41-,43+,44+,45-,46-,47+,48+,52+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability of the compound to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 921-926 (1994)
Article DOI: 10.1016/S0960-894X(01)80264-X
BindingDB Entry DOI: 10.7270/Q2HD7VMG |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284185

(C32-O-cinnamyl ether analogue of L-683590 | CHEMBL...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC(O)([C@@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC(C)C |c:3| Show InChI InChI=1S/C45H73NO12/c1-11-32-19-26(4)18-27(5)20-38(54-9)41-39(55-10)22-29(7)45(53,58-41)42(50)43(51)46-17-13-12-14-33(46)44(52)57-40(30(8)35(48)24-36(32)49)28(6)21-31-15-16-34(47)37(23-31)56-25(2)3/h19,21,25,27,29-35,37-41,47-48,53H,11-18,20,22-24H2,1-10H3/b26-19+,28-21+/t27-,29-,30+,31-,32+,33-,34+,35-,37+,38-,39-,40+,41+,45?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability of the compound to bind the major cystolic receptor FKBP12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 927-930 (1994)
Article DOI: 10.1016/S0960-894X(01)80265-1
BindingDB Entry DOI: 10.7270/Q2CN73VP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284195

(C32-O-cinnamyl ether analogue of L-683590 | CHEMBL...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC(O)([C@@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OCc2ccsc2)[C@H](O)C1 |c:3| Show InChI InChI=1S/C47H71NO12S/c1-9-34-19-27(2)18-28(3)20-40(56-7)43-41(57-8)22-30(5)47(55,60-43)44(52)45(53)48-16-11-10-12-35(48)46(54)59-42(31(6)36(49)24-37(34)50)29(4)21-32-13-14-39(38(51)23-32)58-25-33-15-17-61-26-33/h15,17,19,21,26,28,30-32,34-36,38-43,49,51,55H,9-14,16,18,20,22-25H2,1-8H3/b27-19+,29-21+/t28-,30-,31+,32-,34+,35-,36-,38+,39+,40-,41-,42+,43+,47?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 927-930 (1994)
Article DOI: 10.1016/S0960-894X(01)80265-1
BindingDB Entry DOI: 10.7270/Q2CN73VP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284190

(C32-O-cinnamyl ether analogue of L-683590 | CHEMBL...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC(O)([C@@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OCc1cccs1 |c:3| Show InChI InChI=1S/C47H71NO12S/c1-9-33-20-27(2)19-28(3)21-40(56-7)43-41(57-8)23-30(5)47(55,60-43)44(52)45(53)48-17-11-10-14-35(48)46(54)59-42(31(6)37(50)25-38(33)51)29(4)22-32-15-16-36(49)39(24-32)58-26-34-13-12-18-61-34/h12-13,18,20,22,28,30-33,35-37,39-43,49-50,55H,9-11,14-17,19,21,23-26H2,1-8H3/b27-20+,29-22+/t28-,30-,31+,32-,33+,35-,36+,37-,39+,40-,41-,42+,43+,47?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 927-930 (1994)
Article DOI: 10.1016/S0960-894X(01)80265-1
BindingDB Entry DOI: 10.7270/Q2CN73VP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284188
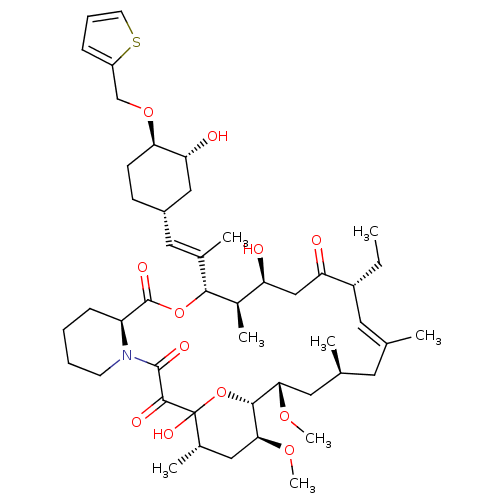
(C32-O-cinnamyl ether analogue of L-683590 | CHEMBL...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC(O)([C@@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OCc2cccs2)[C@H](O)C1 |c:3| Show InChI InChI=1S/C47H71NO12S/c1-9-33-20-27(2)19-28(3)21-40(56-7)43-41(57-8)23-30(5)47(55,60-43)44(52)45(53)48-17-11-10-14-35(48)46(54)59-42(31(6)36(49)25-37(33)50)29(4)22-32-15-16-39(38(51)24-32)58-26-34-13-12-18-61-34/h12-13,18,20,22,28,30-33,35-36,38-43,49,51,55H,9-11,14-17,19,21,23-26H2,1-8H3/b27-20+,29-22+/t28-,30-,31+,32-,33+,35-,36-,38+,39+,40-,41-,42+,43+,47?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 927-930 (1994)
Article DOI: 10.1016/S0960-894X(01)80265-1
BindingDB Entry DOI: 10.7270/Q2CN73VP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284186
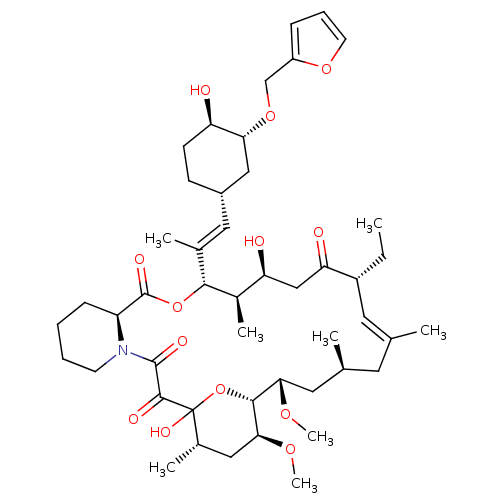
(C32-O-cinnamyl ether analogue of L-683590 | CHEMBL...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC(O)([C@@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OCc1ccco1 |c:3| Show InChI InChI=1S/C47H71NO13/c1-9-33-20-27(2)19-28(3)21-40(56-7)43-41(57-8)23-30(5)47(55,61-43)44(52)45(53)48-17-11-10-14-35(48)46(54)60-42(31(6)37(50)25-38(33)51)29(4)22-32-15-16-36(49)39(24-32)59-26-34-13-12-18-58-34/h12-13,18,20,22,28,30-33,35-37,39-43,49-50,55H,9-11,14-17,19,21,23-26H2,1-8H3/b27-20+,29-22+/t28-,30-,31+,32-,33+,35-,36+,37-,39+,40-,41-,42+,43+,47?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 927-930 (1994)
Article DOI: 10.1016/S0960-894X(01)80265-1
BindingDB Entry DOI: 10.7270/Q2CN73VP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284196

(C32-O-cinnamyl ether analogue of L-683590 | CHEMBL...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC(O)([C@@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OC(C)C=C)[C@H](O)C1 |c:3| Show InChI InChI=1S/C46H73NO12/c1-11-30(7)57-38-17-16-32(24-37(38)50)22-28(5)41-31(8)35(48)25-36(49)33(12-2)20-26(3)19-27(4)21-39(55-9)42-40(56-10)23-29(6)46(54,59-42)43(51)44(52)47-18-14-13-15-34(47)45(53)58-41/h11,20,22,27,29-35,37-42,48,50,54H,1,12-19,21,23-25H2,2-10H3/b26-20+,28-22+/t27-,29-,30?,31+,32-,33+,34-,35-,37+,38+,39-,40-,41+,42+,46?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 927-930 (1994)
Article DOI: 10.1016/S0960-894X(01)80265-1
BindingDB Entry DOI: 10.7270/Q2CN73VP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284187
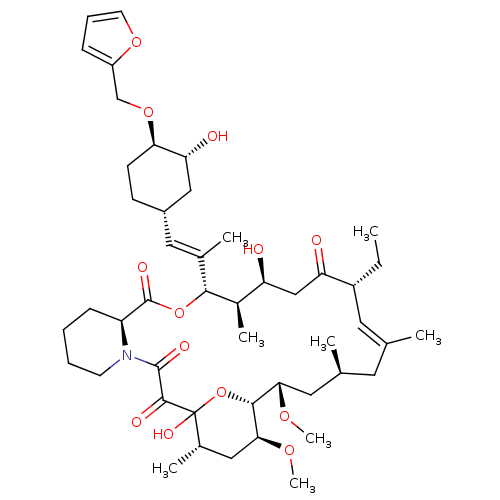
(C32-O-cinnamyl ether analogue of L-683590 | CHEMBL...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC(O)([C@@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OCc2ccco2)[C@H](O)C1 |c:3| Show InChI InChI=1S/C47H71NO13/c1-9-33-20-27(2)19-28(3)21-40(56-7)43-41(57-8)23-30(5)47(55,61-43)44(52)45(53)48-17-11-10-14-35(48)46(54)60-42(31(6)36(49)25-37(33)50)29(4)22-32-15-16-39(38(51)24-32)59-26-34-13-12-18-58-34/h12-13,18,20,22,28,30-33,35-36,38-43,49,51,55H,9-11,14-17,19,21,23-26H2,1-8H3/b27-20+,29-22+/t28-,30-,31+,32-,33+,35-,36-,38+,39+,40-,41-,42+,43+,47?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 927-930 (1994)
Article DOI: 10.1016/S0960-894X(01)80265-1
BindingDB Entry DOI: 10.7270/Q2CN73VP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50068939

((E)-(9S,12S,13R,14S,17R,21S,23S,24R,25S,27S)-17-Et...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2O[C@](O)([C@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C43H69NO12/c1-10-30-18-24(2)17-25(3)19-36(53-8)39-37(54-9)21-27(5)43(51,56-39)40(48)41(49)44-16-12-11-13-31(44)42(50)55-38(28(6)33(46)23-34(30)47)26(4)20-29-14-15-32(45)35(22-29)52-7/h18,20,25,27-33,35-39,45-46,51H,10-17,19,21-23H2,1-9H3/b24-18+,26-20+/t25-,27+,28+,29-,30+,31-,32+,33-,35+,36-,37-,38+,39+,43+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 927-930 (1994)
Article DOI: 10.1016/S0960-894X(01)80265-1
BindingDB Entry DOI: 10.7270/Q2CN73VP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284194

(C32-O-cinnamyl ether analogue of L-683590 | CHEMBL...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC(O)([C@@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC(C)C=C |c:3| Show InChI InChI=1S/C46H73NO12/c1-11-30(7)57-38-24-32(16-17-35(38)48)22-28(5)41-31(8)36(49)25-37(50)33(12-2)20-26(3)19-27(4)21-39(55-9)42-40(56-10)23-29(6)46(54,59-42)43(51)44(52)47-18-14-13-15-34(47)45(53)58-41/h11,20,22,27,29-36,38-42,48-49,54H,1,12-19,21,23-25H2,2-10H3/b26-20+,28-22+/t27-,29-,30?,31+,32-,33+,34-,35+,36-,38+,39-,40-,41+,42+,46?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 927-930 (1994)
Article DOI: 10.1016/S0960-894X(01)80265-1
BindingDB Entry DOI: 10.7270/Q2CN73VP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284193
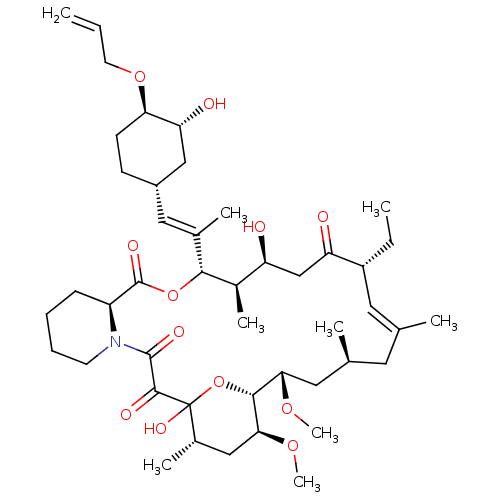
((E)-(9S,12S,13R,14S,17R,21S,23S,24R,25S,27S)-12-[(...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC(O)([C@@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](OCC=C)[C@H](O)C1 |c:3| Show InChI InChI=1S/C45H71NO12/c1-10-18-56-37-16-15-31(24-36(37)49)22-28(5)40-30(7)34(47)25-35(48)32(11-2)20-26(3)19-27(4)21-38(54-8)41-39(55-9)23-29(6)45(53,58-41)42(50)43(51)46-17-13-12-14-33(46)44(52)57-40/h10,20,22,27,29-34,36-41,47,49,53H,1,11-19,21,23-25H2,2-9H3/b26-20+,28-22+/t27-,29-,30+,31-,32+,33-,34-,36+,37+,38-,39-,40+,41+,45?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 927-930 (1994)
Article DOI: 10.1016/S0960-894X(01)80265-1
BindingDB Entry DOI: 10.7270/Q2CN73VP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284197

((E)-(9S,12S,13R,14S,17R,21S,23S,24R,25S,27S)-12-[(...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC(O)([C@@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OCC=C |c:3| Show InChI InChI=1S/C45H71NO12/c1-10-18-56-37-24-31(15-16-34(37)47)22-28(5)40-30(7)35(48)25-36(49)32(11-2)20-26(3)19-27(4)21-38(54-8)41-39(55-9)23-29(6)45(53,58-41)42(50)43(51)46-17-13-12-14-33(46)44(52)57-40/h10,20,22,27,29-35,37-41,47-48,53H,1,11-19,21,23-25H2,2-9H3/b26-20+,28-22+/t27-,29-,30+,31-,32+,33-,34+,35-,37+,38-,39-,40+,41+,45?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 927-930 (1994)
Article DOI: 10.1016/S0960-894X(01)80265-1
BindingDB Entry DOI: 10.7270/Q2CN73VP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50284198
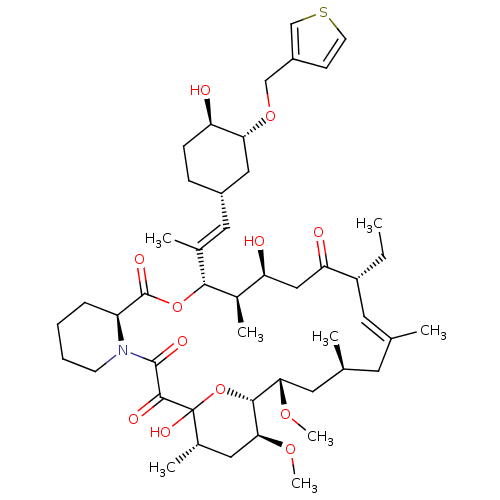
(C32-O-cinnamyl ether analogue of L-683590 | CHEMBL...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC(O)([C@@H](C)C[C@@H]2OC)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OCc1ccsc1 |c:3| Show InChI InChI=1S/C47H71NO12S/c1-9-34-19-27(2)18-28(3)20-40(56-7)43-41(57-8)22-30(5)47(55,60-43)44(52)45(53)48-16-11-10-12-35(48)46(54)59-42(31(6)37(50)24-38(34)51)29(4)21-32-13-14-36(49)39(23-32)58-25-33-15-17-61-26-33/h15,17,19,21,26,28,30-32,34-37,39-43,49-50,55H,9-14,16,18,20,22-25H2,1-8H3/b27-19+,29-21+/t28-,30-,31+,32-,34+,35-,36+,37-,39+,40-,41-,42+,43+,47?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind the major cystolic receptor FK506 binding protein 12 by using competitive binding assay |
Bioorg Med Chem Lett 4: 927-930 (1994)
Article DOI: 10.1016/S0960-894X(01)80265-1
BindingDB Entry DOI: 10.7270/Q2CN73VP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data